Renal Ischemia-Reperfusion (IR) Modeling & Pharmacodynamics Service
Creative Biolabs offers a variety of well-established models to assess the efficacy of drugs in the treatment of acute kidney injury, supporting the development of novel therapies aimed at mitigating renal damage and improving kidney function.
Introduction
Acute kidney injury (AKI) is a rapid decline in renal function, often characterized by a rise in serum creatinine and a reduction in urine output. It can be caused by a variety of factors, including ischemia (reduced blood flow), nephrotoxic drugs, infections, or trauma. AKI is a serious and potentially life-threatening condition, and if left untreated, it can progress to chronic kidney disease (CKD) and end-stage renal failure. The pathophysiology of AKI involves inflammation, oxidative stress, and tubule cell injury, which compromise the kidney's ability to filter waste and maintain fluid-electrolyte balance. Early detection and intervention are essential to improving patient outcomes, making AKI a critical area of research for developing effective treatments. The disease can be categorized into prerenal, intrinsic, and postrenal types, depending on the cause of the injury.
Renal Ischemia-Reperfusion (IR) Model
The Renal Ischemia-Reperfusion (IR) Model is commonly used to study the pathophysiology of kidney injury caused by ischemia followed by reperfusion. This model is typically established by occluding the renal artery for a specified duration, followed by reperfusion to mimic the ischemic damage that occurs in clinical conditions like transplant surgery or renal artery stenosis. The model closely replicates the inflammation, oxidative stress, and renal injury observed in human AKI. Advantages include the ability to evaluate kidney function, histopathology, and response to treatment. However, its limitations lie in the variability of injury severity and potential difficulty in quantifying ischemic damage. Researchers can utilize this model to test potential renal protective agents and therapies aimed at mitigating ischemic damage.
- Simulates: The Renal Ischemia-Reperfusion (IR) Model simulates kidney injury induced by a temporary lack of blood flow followed by the restoration of circulation.
- Evaluates Drugs: The IR model helps test the efficacy of novel pharmacological agents, including renal protective compounds, anti-inflammatory drugs, and molecules targeting the oxidative stress pathways, to prevent or mitigate kidney damage after ischemia and reperfusion.
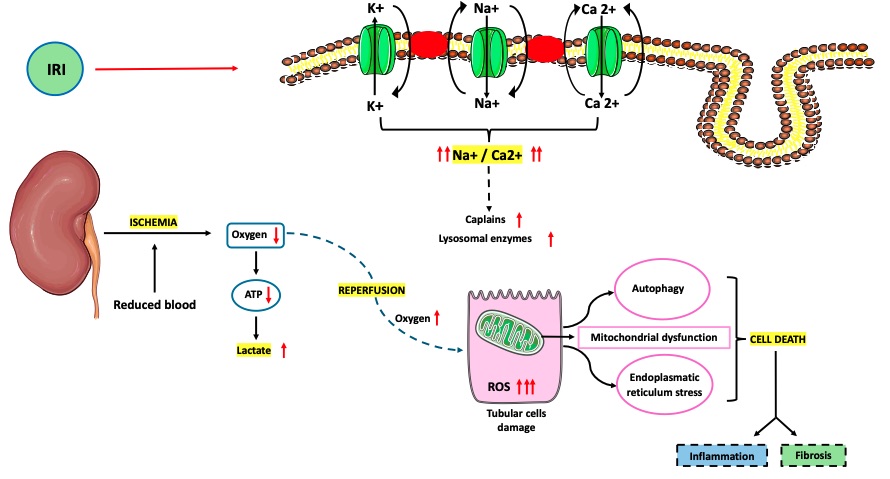 Fig. 1 Pathophysiology of ischemia–reperfusion injury (IRI).1,3
Fig. 1 Pathophysiology of ischemia–reperfusion injury (IRI).1,3
Evaluation Platform
- Animals: Mouse, Rat, Rabbit, NHPs.
-
Measurements
We offer a variety of measurements for assessing drug efficacy in renal ischemia-reperfusion models, leveraging advanced technologies such as:- General observations: Body weight, urine output, renal function markers (creatinine, BUN), and mortality rate.
- Histopathology: Renal tissue analysis using H&E staining to assess tubular necrosis, inflammation, and overall tissue integrity.
- Immunohistochemistry: Detection of immune cell infiltration (e.g., macrophages, T-cells) in renal tissue.
- Cytokine profiling (ELISA): Measurement of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β to assess inflammation levels.
- Oxidative stress markers: Quantification of reactive oxygen species (ROS), malondialdehyde (MDA), and antioxidant enzyme activity (e.g., SOD, CAT).
- Gene/protein expression profiling: RT-qPCR and Western blot for evaluating the expression of genes and proteins involved in cell survival, apoptosis, and inflammation.
In addition to established methods, our team can assist in custom experimental designs to meet specific research needs, ensuring accurate and reproducible results.
Related Services
We also offer other animal models to induce acute kidney injury, including but not limited to models involving glycerol, cisplatin, and folic acid. These models complement our renal ischemia-reperfusion studies and broaden your research scope.
- Cecal Ligation and Puncture (CLP)-Induced Acute Kidney Injury Model
- Lysophosphatidic acid (LPA)-Induced Acute Kidney Injury Model
- Growth Hormone-Releasing Hormone (GHRH)-Induced Acute Kidney Injury Model
- Anti-TSHR Antibody-Induced Acute Kidney Injury Model
- Aristolochic Acid A-Induced Acute Kidney Injury Model
Our advantages
- High Relevance to Clinical Conditions: The Renal Ischemia-Reperfusion (IR) model closely mimics real-world scenarios like kidney transplantation and ischemic nephropathy, making it highly relevant for studying kidney injury and therapeutic interventions.
- Wide Range of Applications: It can be used to test various drug classes, including anti-inflammatory, antioxidant, and cytoprotective agents, making it versatile for both basic research and drug development.
- Customizable Protocols: The model can be tailored to replicate different levels of ischemic injury, allowing for the study of mild to severe forms of kidney damage and testing drugs with varying mechanisms of action.
- Reliable and Reproducible Results: The model has been extensively validated and widely used, ensuring consistent and reproducible results for assessing kidney injury and recovery.
- Comprehensive Monitoring: It provides a broad spectrum of assessment methods, including histopathology, kidney function markers, and molecular analyses, offering a complete picture of drug efficacy.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the typical duration for inducing AKI in the Renal Ischemia-Reperfusion model?
The ischemic period generally ranges from 30 minutes to 1 hour, depending on the severity of injury being studied.
-
2. Can I use this model to test kidney protective agents?
Yes, this model is ideal for evaluating drugs aimed at reducing kidney damage following ischemia and reperfusion.
-
3. What kind of tissue analysis can be performed on kidneys from this model?
Histological analysis using H&E staining, immunohistochemistry, and molecular profiling can be performed to assess tissue damage, inflammation, and cellular responses.
Published Data
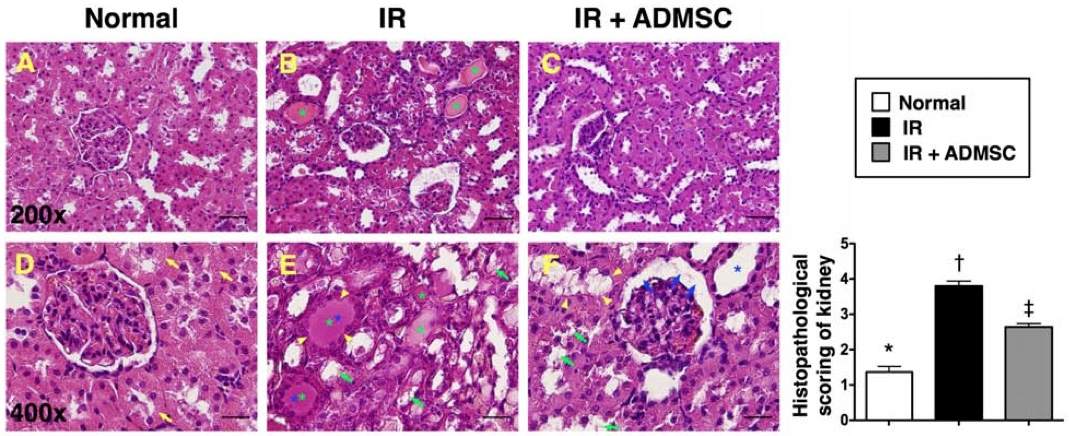 Fig. 2 Histopathological scoring of ischemia-reperfusion (IR)-induced renal injury.2,3
Fig. 2 Histopathological scoring of ischemia-reperfusion (IR)-induced renal injury.2,3
To assess the effects of ADMSC transplantation on renal injury induced by ischemia-reperfusion (IR), histopathological scoring was performed based on characteristic microscopic features of acute tubular damage. These included extensive tubular necrosis, tubular dilatation, cast formation, and loss of the brush border (Figure 2). The histological examination revealed more severe kidney injury in group 2 compared to group 3, indicating that ADMSC therapy provided significant protection against IR-induced renal damage. This analysis highlights the potential of ADMSC as a therapeutic strategy for mitigating ischemic kidney injury.
References
- Lasorsa, Francesco et al. "Ischemia-Reperfusion Injury in Kidney Transplantation: Mechanisms and Potential Therapeutic Targets." International Journal of Molecular Sciences vol. 25,8 4332. 14 Apr. 2024. https://doi.org/10.3390/ijms25084332
- Chen, Yen-Ta et al. "Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction." Journal of Translational Medicine vol. 9 51. 5 May. 2011. https://doi.org/10.1186/1479-5876-9-51
- Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
