Cyclophosphamide (CYP) induced Cystitis Modeling & Pharmacodynamics Service
Creative Biolabs offers a variety of well-established models to evaluate the efficacy of drugs targeting cystitis. These models can help in screening anti-inflammatory agents, bladder-protective drugs, and treatments aimed at reducing the severity of cystitis symptoms and preventing long-term bladder damage. Through these models, we provide comprehensive drug evaluation services to advance bladder disease research.
Introduction
Cystitis is an inflammation of the bladder, often associated with symptoms such as urinary frequency, urgency, discomfort, and in some cases, hematuria (blood in the urine). It can be caused by various factors, including bacterial infections, chemical irritants, and certain medications like cyclophosphamide, a chemotherapeutic agent. Chemotherapy-induced cystitis is a common complication in cancer patients and often leads to bladder damage, pain, and bleeding. The condition can range from mild irritation to severe, hemorrhagic cystitis, potentially causing long-term complications such as bladder fibrosis and reduced bladder function. There are several types of cystitis, with bacterial cystitis being the most common, particularly among women. However, cyclophosphamide (CYP)-induced cystitis represents a significant model for studying bladder inflammation and evaluating drug efficacy. This model mimics the toxic and inflammatory effects of chemotherapy drugs, making it valuable for testing potential treatments for bladder-related disorders.
Cyclophosphamide (CYP)-Induced Cystitis Model
The Cyclophosphamide (CYP)-Induced Cystitis Model is created by administering a single dose of cyclophosphamide to rodents, which induces severe bladder inflammation, including hemorrhagic cystitis. This model exhibits pathological changes such as bladder wall thickening, urothelial damage, and leukocyte infiltration, mimicking the clinical features of cystitis in humans. It's particularly useful for studying the effects of anti-inflammatory and anti-fibrotic agents on bladder health. However, its limitation lies in the fact that the model primarily focuses on the inflammatory response and may not fully replicate the underlying causes of all cystitis forms, such as infection-induced cystitis. Despite this, it remains a valuable tool for drug screening and mechanistic studies.
- Simulates: This model simulates chemotherapy-induced cystitis, particularly the inflammation and hemorrhagic conditions associated with cyclophosphamide treatment.
- Evaluates Drugs: The model evaluates drugs aimed at reducing bladder inflammation, promoting tissue healing, and mitigating side effects such as hemorrhagic cystitis.
Evaluation Platform
- Animals: Mouse, Rat, Hamster, Rabbit, Cat, Dog, NHPs.
-
Measurements
We offer a variety of measurements for evaluating drug efficacy in the Cyclophosphamide-Induced Cystitis (CYP) Model, utilizing a range of advanced technologies, including but not limited to:- General Observations: Body weight, mortality rate, urinary frequency, hematuria (blood in urine), and overall health status of the animals.
- Histopathological Analysis: Evaluation of bladder tissue damage, including bladder wall thickness, urothelial injury, and the degree of inflammatory infiltration (e.g., neutrophils, macrophages).
- Cytokine Profiling (e.g., ELISA): Measurement of inflammatory cytokines such as TNF-α, IL-1β, IL-6, and other markers of bladder inflammation.
- Urinary Biomarkers: Detection of urinary biomarkers such as proteinuria, hematuria, and other kidney function indicators.
- Immunohistochemistry: Infiltration of immune cells in bladder tissues, including T-cells, macrophages, and neutrophils, to assess inflammatory responses and tissue repair processes.
- Gene/Protein Expression Profiling: Quantification of gene and protein expression associated with inflammation and bladder tissue damage using RT-qPCR and Western blotting techniques.
- Bladder Function Assessment: Measurement of bladder capacity and voiding behavior to evaluate functional impairment and recovery.
In addition to these established evaluation techniques, our expertise extends to the development of novel animal models specifically tailored to your research needs. Our scientific team is available to assist with experimental design, model selection, and data analysis, ensuring a customized approach to your project at every stage.
Related Services
In addition to the Cyclophosphamide-Induced Cystitis Model, we provide other models of cystitis induced by agents like lipopolysaccharides (LPS) and bacterial infections. Each model offers unique advantages based on the type of bladder inflammation being studied, and our team is ready to assist in choosing the most suitable model for your research.
Lipopolysaccharide (LPS)-Induced Cystitis Model
Our advantages
- Customization: Tailored services based on specific research goals, ensuring relevant outcomes for your study.
- Comprehensive Support: From experimental design to data analysis, our team provides ongoing scientific guidance.
- Advanced Technology: Utilization of cutting-edge techniques for accurate measurement and evaluation.
- Expertise: Over a decade of experience in establishing and managing rodent disease models.
- Reproducibility: Well-established protocols ensuring consistent and reproducible results.
Work with Us
- Summarize the project requirements and fill in the information collection form.
- Sign a CDA from both parties to further communicate information, such as targets.
- Select an animal model, discuss experimental design, and determine assay parameters.
- Project costing and project schedule forecasting.
- We provide a detailed project plan, including the required sample quantities, methods, and protocols.
- Both parties confirm the project details and start the project.
- Confirm the timeline of the project.
- We provide periodic results and information on the animal's condition.
- We will work together to make project adjustments as necessary.
- We provide a comprehensive project report promptly.
- We arrange transportation for the produced samples.
- We provide a discussion of the project results and help to arrange the next steps.
- Data storage and archiving.
FAQs
-
1. What is the typical dosing schedule for cyclophosphamide in this model?
The standard dosing involves a single dose of cyclophosphamide, typically administered intraperitoneally. The exact dose and schedule can be customized based on the study's goals.
-
2. Can this model be used for testing combination therapies?
Yes, the model is ideal for evaluating the efficacy of combination therapies, including anti-inflammatory agents alongside drugs targeting bladder tissue repair.
-
3. How long do the symptoms of cystitis last in the model?
Symptoms usually peak within 24-48 hours post-administration and can last for up to a week, depending on the treatment and model protocol.
-
4. Can this model be used to assess the long-term effects of cystitis treatments?
Yes, by using a chronic dosing regimen or evaluating tissue recovery over extended periods, long-term treatment effects can be assessed.
Published Data
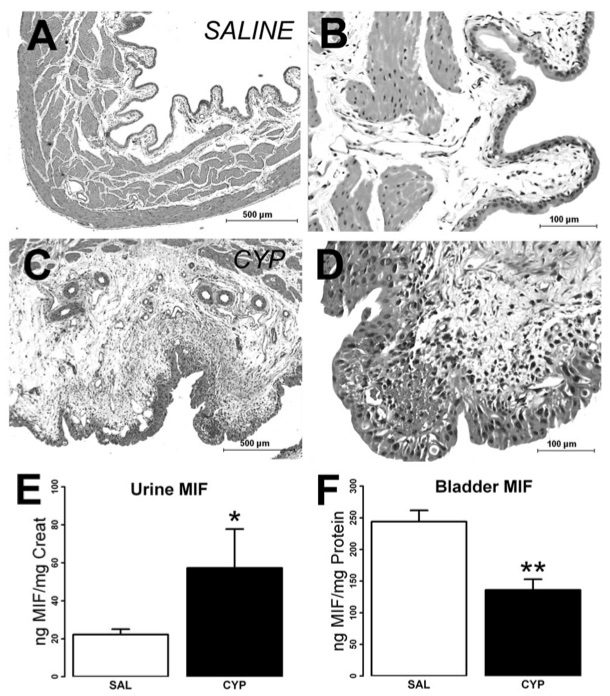 Fig. 1 Effect of cyclophosphamide (CYP) treatment on bladder histology, bladder, and urine MIF levels. 1
Fig. 1 Effect of cyclophosphamide (CYP) treatment on bladder histology, bladder, and urine MIF levels. 1
Histological examination of H&E-stained bladder sections from saline-treated rats revealed normal bladder morphology (Figure 1A, B). In contrast, bladder tissues from CYP-treated rats exhibited significant signs of inflammation, including submucosal edema, urothelial disruption, cellular infiltration, hemorrhage, and fibroblast proliferation (Figure 1C). While the urothelium in saline-treated rats maintained its characteristic three-layer structure (Figure 1B), CYP treatment induced urothelial hyperplasia (Figure 1D). Furthermore, CYP treatment led to a marked increase in urinary MIF levels compared to saline treatment. This experimental model effectively demonstrates the inflammatory and pathological changes induced by cyclophosphamide, providing valuable insights into the progression of cystitis.
Reference
- Vera, Pedro L et al. "Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association." PloS one vol. 3,12 (2008): e3898. Distributed under Open Access license CC BY 4.0, without modification. https://doi.org/10.1371/journal.pone.0003898
For Research Use Only.
