GTOnco™ Mixed Lymphocyte Tumor Reactivity Assay
It is vital to determine the immune reactivity of lymphocytes and tumor cells from the same individual to support the gene therapy-based I-O drugs development. Creative Biolabs now presents one-stop mixed lymphocyte tumor cell (MLTuC), also called the mixed lymphocyte tumor reactivity (MLTR) assay, to evaluate the efficiency of I-O products. MLTR assay is a method to assess the immunological reactivity between lymphocytes and tumor cells, such as the induction of primary immune responses to tumor-associated cell-surface antigens. In addition, the assay can be used to generate T-lymphocytes that are cytotoxic to specific tumor cell types. At GTOnco™, we are committed to offering the MLTR assay for our clients to advance their I-O drugs development process.
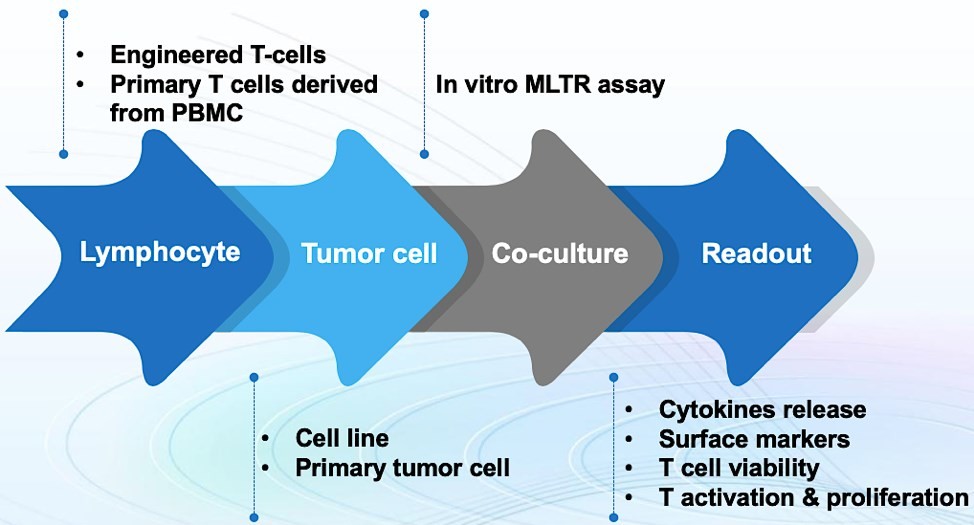 Figure 1. Simple diagram of MLTR assay at GTOnco™.
Figure 1. Simple diagram of MLTR assay at GTOnco™.
What is Mixed Lymphocyte Tumor Reactivity Assay?
MLTR assay is an advanced in vitro technology designed to quantitatively measure the potency and specificity of lymphocyte-mediated responses against tumor cells. This sophisticated platform recapitulates key aspects of the tumor-immune interface, enabling researchers to study the complex dynamics between immune effector cells and malignant targets under controlled conditions. The basic principle of the MLTR assay involves co-culturing autologous or allogeneic lymphocytes with tumor cells, followed by assessment of various parameters of immune activation, including cytokine secretion, cytotoxic activity, and proliferative responses. This approach provides valuable insights into the function of tumor-infiltrating lymphocytes and peripheral blood mononuclear cells in the face of cancer cells, serving as a predictive tool for assessing anti-tumor immunity.
Comparative Analysis Between Gtonco™ MLTR Assay And Traditional Methods
| Parameter | GTOnco™ MLTR Assay | Traditional Methods (e.g., ELISA, Chromium-Release Assay) |
|---|---|---|
| Complexity of immune-tumor interactions | Recapitulates complex tumor microenvironment with multiple cell types | Typically measures single endpoints in simplified systems |
| Temporal resolution | Allows continuous monitoring of immune responses over time | Provides single timepoint measurements |
| Physiological relevance | Maintains physiological oxygen tension and includes tumor stroma components | Often uses non-physiological oxygen levels and pure tumor cell lines |
| Quantitative output | Multiplexed readouts provide quantitative data on multiple parameters | Generally limited to one or two readouts with variable quantification |
| Throughput | Medium throughput with capacity for limited screening | Variable throughput, often low when using primary human cells |
| Standardization | Highly standardized protocols with strict quality controls | Variable standardization across laboratories |
| Resource requirements | Requires specialized equipment and technical expertise | Generally less resource-intensive |
MLTR Assay in Tumor Immunogenicity Assessment
In the era of cancer immunotherapy, particularly with the widespread use of immune checkpoint inhibitors (ICIs), the clinical treatment landscape for cancer has fundamentally changed. Traditional biomarkers, such as tumor mutational burden (TMB) and microsatellite instability (MSI), while providing some guidance, are inherently static genomic indicators that fail to fully capture the dynamic functional response of the host immune system to tumors. Assessing tumor immunogenicity—the ability of a tumor to elicit a specific anti-tumor immune response—has become crucial for developing next-generation predictive models. The mixed lymphocyte reaction (MLR) has historically been the gold standard for assessing allogeneic or autologous immune responses in vitro. The MLTRA is a highly optimized and standardized functional assessment platform developed based on this platform. It aims to overcome the inherent limitations of traditional MLR in oncology applications, enabling precise and quantitative assessment of patient T cell specificity for autologous tumor antigens.
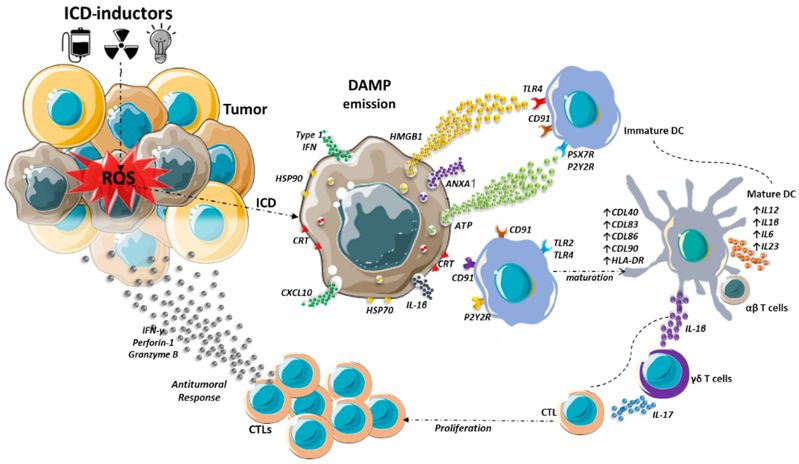 Figure 1. Engagement of adaptive immune response after immunogenic cell death in a tumor.1
Figure 1. Engagement of adaptive immune response after immunogenic cell death in a tumor.1
Core Services at Creative Biolabs
Creative Biolabs offers a comprehensive suite of GTOnco™ MLTRA services to meet the needs of academic researchers, biotech companies, and pharmaceutical companies. Our services are designed to accelerate research timelines, ensure data quality, and support regulatory compliance.
| Service Tier | Key Features |
|---|---|
| Customized MLTR Assay Design | Customize services based on the nature of treatment and research objectives (such as efficacy, MOA, immunogenicity). |
| Advanced MLTRA | Custom readouts (e.g., cytokine panel, high-content imaging for organoids, immune migration assay). |
| Standardized Assay | This service includes the comprehensive implementation of GTOnco™ MLTR testing and providing complete readings (cytotoxicity, cytokine secretion, proliferation, and activation markers) in a comprehensive report format. |
MLTR Assay at GTOnco™ Platform
Generally, the MLTR assay is performed by the co-culture of targeted tumor cells with lymphocyte populations, including engineered T-cells or primary T cells derived from PBMC. GTOnco™ provides multiple technologies to assess the T cell viability, activation, proliferation, and cytotoxicity after incubated with tumor cells. Additionally, in order to generate T-lymphocytes with enhanced anti-tumor activity, we conduct the MLTR assay via the addition of dendritic cells to present tumor antigens of interest to T-lymphocytes in culture. All our experiments are performed by well-trained and experienced technicians to guarantee quality and reliability.
Applications of the GTOnco™ Mixed Lymphocyte Tumor Reactivity Assay
The versatility and depth of functionality of the MLTR assay make it a key tool in multiple areas of I/O and translational research:
Potency Testing of T Cell Therapies (CAR-T/TCR-T)
It is crucial for determining the in vitro functional activity and dose-response characteristics of genetically engineered T cells. This is often a regulatory requirement for Investigational New Drug (IND) applications.
Immunomodulatory Drug Screening
Evaluating the mechanism of action (MOA) of novel agents, such as immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4) or co-stimulatory agonists, by measuring their ability to enhance or inhibit T cell tumor responses.
Biomarker Discovery and Patient Stratification
Identifying relevant biomarkers (e.g., specific cytokine profiles or T cell phenotypes) that predict clinical response to immunotherapy. This data is crucial for geographic, ethnic, and disease-specific (GEO) analyses, helping to stratify patient populations for clinical trials.
Vaccine Development
Evaluating the immunogenicity of therapeutic cancer vaccines by measuring T cell activation and proliferation induced by vaccine-derived antigens in the presence of APCs and patient-derived lymphocytes. Immunotoxicity/Safety Assessment: Serving as an initial screen for potential T-cell hyperactivation (e.g., risk of cytokine release syndrome) by measuring excessive proliferation and cytokine production in response to treatment.
Features of Our MLTR Assay at GTOnco™ Platform
- Skillful technologies and advanced platforms
- Customized assay design to support per clients' project
- Multiple endpoints are available to clearly understand the I-O profile, including surface markers, cytokines release, T cell activation and proliferation, etc.
Workflow of Our MLTR Assay
Creative Biolabs has streamlined the GTOnco™ MLTRA workflow to ensure efficiency, transparency, and customer satisfaction. From order placement to results delivery, the entire process typically takes 2-4 weeks (depending on service level), with clear milestones and communication at each step:
-
Step 1
Initial Consultation
- Customers submit sample details (tumor type, sample type, desired readout) through our online portal or email.
- Our team of immunologists reviews the request, provides a feasibility assessment, and recommends assay parameters (e.g., co-culture ratio, incubation time).
- A detailed quote and project timeline are sent to the customer.
-
Step 2
Sample Shipping and Receipt
Customers ship their samples in our pre-labeled, temperature-monitored packaging (dry ice is used for frozen samples). Our laboratory confirms receipt of the sample within 24 hours, tests for viability, and notifies the customer of sample quality. If the sample does not meet viability criteria, we work with the customer to troubleshoot (e.g., adjust shipping conditions).
-
Step 3
Assay Execution
Laboratory technicians perform sample preparation (tissue digestion, lymphocyte isolation) and co-culture setup according to the approved protocol. Interim data is shared with the customer to ensure smooth trial execution. Readouts are measured using flow cytometry or high-content imaging, with quality control checks performed at every step.
-
Step 4
Data Analysis and Report Delivery
- Raw data are analyzed using GTOnco™ Analytics, and a comprehensive report (including methods, results, statistical analysis, and interpretation) is generated.
- The report is reviewed for accuracy by a senior scientist before being sent to the client.
- A post-delivery consultation is planned to allow the client to review the results and address any questions.
Frequently Asked Questions
Q: GTOnco™ What cell types can be used in MLTR detection?
A: This assay is compatible with various lymphocyte sources (PBMC, TIL, or specific T cell subpopulations) and tumor cells (established cell lines, patient derived xenografts, or primary tumor cells). The optimal combination depends on the specific research question, and our team can provide selection guidance based on project objectives.
Q: GTOnco™ What is the difference between MLTR testing and traditional cytotoxicity testing?
A: Unlike traditional tests that typically measure a single endpoint, such as chromium release, GTOnco™ platform integrates multiple readings to provide a more comprehensive assessment of immune function. In addition, maintaining greater physiological relevance through specialized cultivation conditions has improved the clinical predictive value of the generated data.
Q: How many experimental conditions can be accommodated in one testing run?
A: The standard platform can evaluate up to 20 different conditions in one run, including appropriate controls. For larger filtering projects, we can implement modified formats to improve throughput while maintaining data quality.
Q: What measures have been taken to ensure data quality and reproducibility?
A: We implement strict quality control measures at multiple stages, including cell viability and purity assessments, standardized protocols with minimal technical differences, appropriate experimental controls, and validation of key reagents. These measures collectively ensure the generation of reliable and reproducible data.
Reach Out to Us Now!
Leveraging our extensive experience and advanced platforms, Creative Biolabs is dedicated to providing one-stop MLTR assay service for our clients to rapidly identify the gene therapy-based I-O agents that modulate antigen presenting cell-mediated lymphocyte response and assess the immunological reactivity between lymphocytes and tumor cells. Please contact us and let us know your requirements and we will get back to you as soon as possible.
Reference
- Rodrigues M C, Morais J A V, Ganassin R, et al. An overview on immunogenic cell death in cancer biology and therapy. Pharmaceutics, 2022, 14(8): 1564. https://doi.org/10.3390/pharmaceutics14081564 (Distributed under Open Access license CC BY 4.0, without modification.)
