GTOnco™ Redirected Cytotoxic Activity Assay
Gene therapy-based I-O products including the bispecific antibodies and chimeric antigen receptor T cells (CAR-T) have been used for the treatment of various malignancies. These approaches take advantage of T-cell potency in cancer therapy by redirecting them against tumors. Importantly, unlike traditional therapies, CAR-T and bispecific antibody approaches circumvent the TCR/HLA-peptide restriction by creating artificial T cell recognition of more general tumor-associated antigens (TAA). Based on advanced technology and years of research, Creative Biolabs has the ability to conduct high-quality redirected cytotoxic activity assay to test the lymphocyte anti-tumor activity. We offer one-stop services for our clients in the redirection of T cells with bispecific antibodies (CD3, CD19) or CAR.
Introduction
The Paradigm Shift in Oncology Treatment
In recent years, cancer treatment has shifted from traditional chemotherapy and radiotherapy to targeted therapies and immunotherapy. The success of immune checkpoint inhibitors (such as PD-1/PD-L1 antibodies) has spurred demand for more aggressive therapies, with the targeted cytotoxicity redirection mechanism becoming a leading focus. This mechanism centers on the use of engineered therapeutics (such as CAR-T cells) to direct and activate powerful immune effector cells to attack target cells expressing specific tumor antigens.
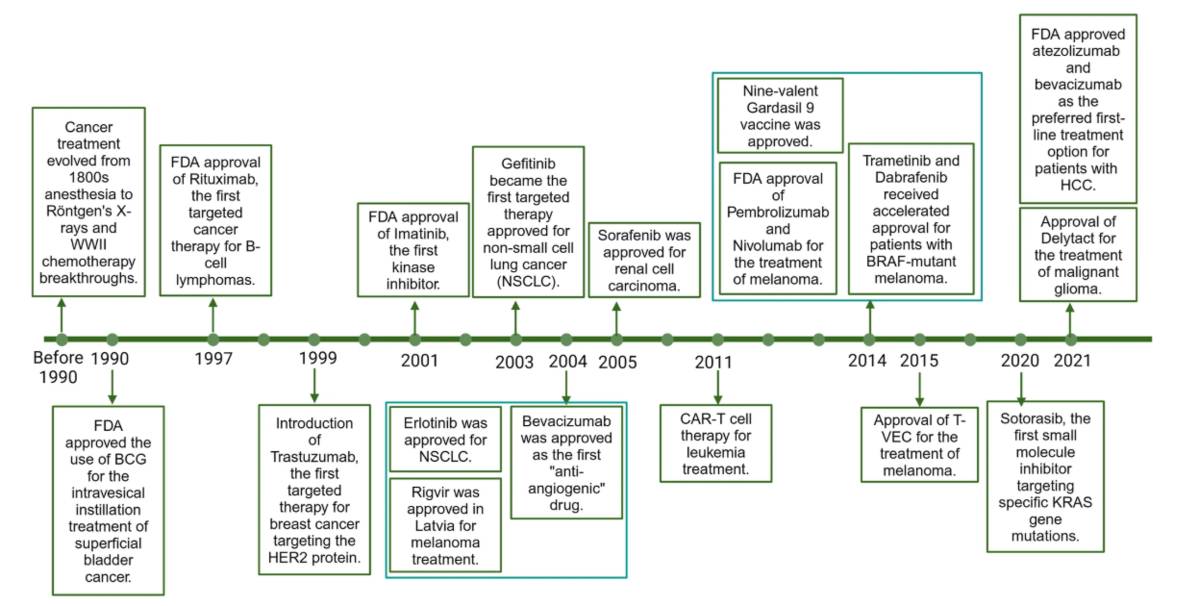 Figure 1 The milestone of cancer therapy development.1
Figure 1 The milestone of cancer therapy development.1
Mechanisms of Redirected Cytotoxicity
Redirected cytotoxicity involves a complex, multistep process:
- Bridging: The therapeutic agent (e.g., a BsAb) connects a coreceptor (e.g., CD3) on the effector cell to a tumor antigen on the target cell.
- Activation: The effector cell becomes activated, rapidly proliferates, and releases cytolytic granules (e.g., perforin and granzyme B).
- Lysis: The target cell is lysed by the effector cell, resulting in apoptosis or necrosis.
Methodology of the Redirected Cytotoxic Activity Assay
- Chromium Release Assay: Historically considered the gold standard, this technique quantifies lysis by monitoring the release of radioactive chromium absorbed by target cells. Advantages: High sensitivity; Disadvantages: Involves radioactive handling.
- Lactate Dehydrogenase Release Assay: When target cell membrane integrity is compromised, intracellular LDH enzyme is released into the culture medium, generating a colorimetric or fluorescent signal through an enzymatic reaction. This method is rapid, safe, and suitable for high-throughput screening.
- Fluorescence-Based Real-Time Cytotoxicity Assay: Target cells are pre-labeled with a non-toxic fluorescent dye. Target cell counts are continuously monitored in a live cell imaging system, eliminating the need for labeling. Key Advantage: This platform provides kinetic data on cell killing, revealing the rate and duration of lysis.
- Phenotypic Analysis: Simultaneously monitors changes in effector cell activation markers, enabling the causal relationship between cell killing activity and effector cell activation.
Strategic Applications of Redirected Cytotoxic Activity Assay in Biomedical Discovery
- Precision potency assays: Compare functional differences between multiple antibody variants or CAR constructs. Molecules with higher potency are generally prioritized for development.
- Specificity validation: Use of antigen-negative cell lines or negative controls with blocking antibodies ensures that cytolytic activity is strictly dependent on the target antigen, thereby reducing the risk of off-target effects.
- On-target/off-tumor safety assessments: In preclinical settings, RCAAs must be used to test the toxicity of therapeutics in healthy primary cells that express low levels of the target antigen. This can predict potential off-tumor toxicity.
Evolution of Redirected Cytotoxic Activity Assay
The evolution of cytotoxicity assays reflects the increasing sophistication of cancer immunotherapy itself. Early methods borrowed from traditional immunology, such as the chromium-51 release assay, while providing fundamental insights, also suffer from limitations such as the use of radioactive materials, spontaneous release, and single-endpoint measurement. The development of nonradioactive alternatives, including the LDH release assay and the MTT metabolic assay, has led to significant progress, but understanding the kinetics and mechanisms of target cell killing remains limited.
Core Services at Creative Biolabs
In the process of natural T cell recognition of tumor cell, the TCRs identify the tumor-derived peptides presented by HLA on the tumor cell surface, promoting T cell activation and the release of cytotoxic mediators. Generally, the approaches are based on the redirection of T cells, for instance, the CD3 bispecific antibody circumvents HLA restriction by simultaneously cross-linking a CD3 component of the T cell receptor complex with a TAA on the surface of the target tumor cell. These methods achieve T cell activation by mimicking the cognate TCR/HLA-peptide interaction.
At GTOnco™, we have developed systematic approaches to assess the redirected cytotoxic activity. In our assay process, the human unstimulated T cells isolated from PBMCs will be co-cultured with the I-O drug treated tumor cells to determine the redirection of T cells and assess the tumor lytic activity of I-O drug in the presence of human T cells. We provide a panel of positive cancer cell lines to confirm the anti-tumor activity and the ration of co-cultured T cells and targeted tumor cells will be also optimized in the assay.
GTOnco™ Platform Advantages and Highlights
Multidimensional Cytotoxicity Profiling
Unlike traditional assays that measure a single cell death parameter, the GTOnco™ platform simultaneously tracks multiple cell death pathways in real time, including apoptosis, necrosis, and pyroptosis.
Advanced Assays
Our platform integrates multiple assay technologies to enhance accuracy under diverse experimental conditions. Our approach combines the strengths of the LDH and MTT assays while addressing their respective limitations through proprietary algorithms.
Physiologically Relevant Microenvironment
The GTOnco™ platform incorporates a 3D microfluidic culture system that closely mimics the tumor microenvironment, including oxygen gradients, extracellular matrix composition, and spatial constraints, more accurately reflecting the in vivo environment.
Our Collaborative Process
-
Phase 1
Comprehensive Consultation
Our process begins with an in-depth consultation designed to understand your specific therapeutic approach, development stage, and key scientific questions. This phase typically includes reviewing existing data, discussing regulatory requirements, and identifying key success factors.
-
Phase 2
Assay Configuration and Optimization
For each new client project, we conduct a limited optimization phase to adapt our standardized protocol to your specific effector and target cells. This may include titrating the effector to target ratio, determining the optimal assay duration, and selecting appropriate endpoint measurement methods.
-
Phase 3
Experimental Execution and Quality Control
During the experimental phase, we continuously monitor quality by incorporating reference standards, system suitability controls, and replicate protocols to ensure data integrity. As the experiment progresses, clients receive regular progress updates and preliminary data reviews.
-
Phase 4
Advanced Data Analysis and Interpretation
Our analytical approaches go beyond simple quantification of specific lysis to encompass kinetic parameters, dose-response models, and multivariate evaluation of multiple readouts. Proprietary bioinformatics tools extract the most valuable information from complex data sets.
-
Phase 5
Reporting and Strategic Consulting
The final deliverable is a comprehensive report that not only presents the experimental results but also contextualizes them within your development project. Our team will strategically interpret the research findings and provide recommendations for next steps in development.
Frequently Asked Questions
Q: What is the best cytotoxicity assay for screening CAR-T drug candidates?
A: The optimal assay depends on your specific development stage and question. For early screening of multiple drug candidates, our high-throughput LDH release assay provides rapid, quantitative, and highly reproducible data. For mechanistic studies of lead drug candidates, our multiplexed fluorescence imaging platform provides unparalleled insights into kinetic parameters and death pathways. We recommend consulting with our technical team during experimental design to select the assay configuration best suited for your specific application.
Q: How does the GTOnco™ platform address inter-donor variability in primary cell experiments?
A: We employ a modular normalization strategy, including an internal reference control in every experiment, enabling meaningful comparisons across donors and experimental batches. Our proprietary inter-donor comparability algorithm further enhances data interpretation by accounting for inherent biological variability. For pivotal studies, we recommend testing multiple donor pairs to determine the robustness of the drug candidate.
Q: Can the GTOnco™ platform be adapted to non-standard effector/target combinations?
A: Of course. Our platform is carefully designed with modularity and flexibility as core principles. We have successfully developed customized cytotoxicity assays for a variety of rare cancer types and unconventional immune cell populations. Our scientific team will work closely with you to adapt our standardized protocols to your specific experimental system.
Q: How does this platform ensure data quality and reproducibility?
A: We implement a multi-level quality control system, including reference standard verification, instrument calibration verification, intra-assay controls, and cross-assay normalization. All assays are rigorously validated according to quality standards and are accompanied by comprehensive performance verification reports. This systematic approach typically achieves inter-assay coefficients of variation (CVs) below 10%, significantly exceeding industry standards.
Connect with Us Anytime!
As a frontier biotech service provider, Creative Biolabs is dedicated to providing the best-characterized in vitro assay service for our clients' gene therapy-based I-O products development. All tests are conducted by experienced technicians with the most advanced techniques. From the foremost project design to the final data interpretation, we implement strict inspections and validations on each and every step. Please feel free to contact us for further discussion with our scientists. Looking forward to cooperating with you.
Reference
- Liu B, Zhou H, Tan L, et al. Exploring treatment options in cancer: tumor treatment strategies. Signal transduction and targeted therapy, 2024, 9(1): 175. https://doi.org/10.1038/s41392-024-01856-7 (Distributed under Open Access license CC BY 4.0, without modification.)
