GTOnco™ Immune Cell-mediated Tumor Lysis Assay Service
Leveraging our extensive expertise in the anti-tumor effect study, Creative Biolabs provides analysis and interpretation of the effects of gene therapy-based I-O candidates for T cell-mediated tumor cells lysis. At GTOnco™, we are able to conduct powerful in vitro immune cell-mediated tumor lysis assay to validate the antitumor activity of lymphocytes. The co-cultured ratio of targeted tumor cells and lymphocytes will be optimized in the assay process. In addition, the targeted tumor cells are also labeled and monitored at a real-time level to measure the tumor cell lysis. We are pleased to share our previous experiences and work closely with our clients to tailor the assays to best address their requirements.
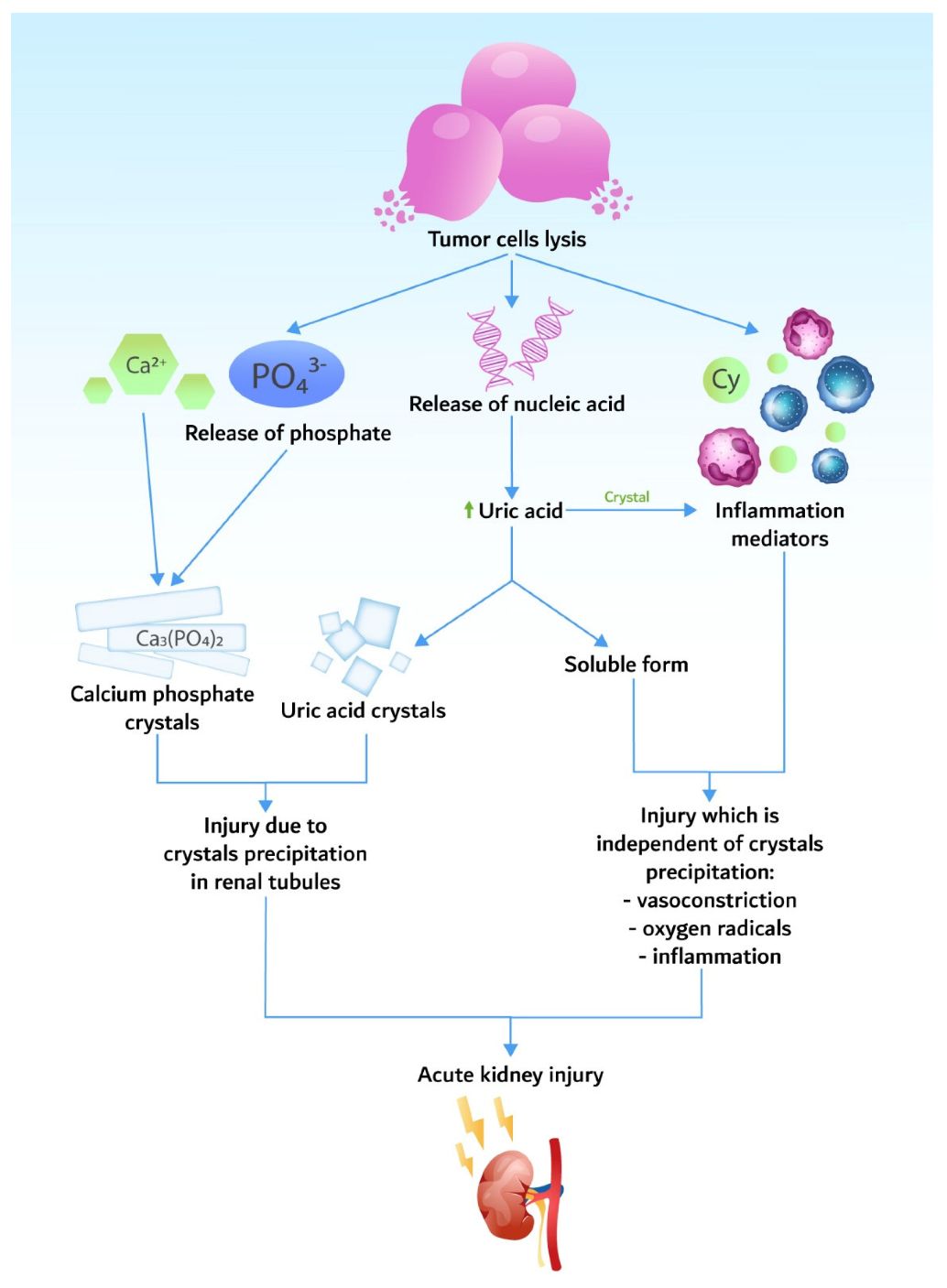 Figure 1 Pathogenesis of tumor lysis syndrome, resulting in acute kidney injury.1
Figure 1 Pathogenesis of tumor lysis syndrome, resulting in acute kidney injury.1
What is Immune Cell-mediated Tumor Lysis Assay?
In the rapidly evolving field of cancer immunotherapy, accurately assessing the function of immune cells against malignant cells has become crucial. Immune cell-mediated tumor lysis assays are a key technology platform for quantifying the cytotoxic capacity of immune effector cells to recognize and eliminate tumor targets. These assays provide valuable insights into the mechanisms of tumor cell killing and are important tools for basic research and the development of novel immunotherapeutic approaches. With the increasing emphasis on immunotherapies in oncology, including CAR-T cells therapy, bispecific antibodies, and immune checkpoint inhibitors, there is a pressing need for robust, reproducible, and predictive in vitro systems that can accurately measure the potency and specificity of immune cells.
Immune Cells in Tumor Lysis
Immune cells are composed of a variety of specialized mediators that orchestrate the body's defense mechanisms against malignant transformation. In tumor immunity, several key cells demonstrate the ability to directly recognize and eliminate cancer cells through diverse molecular mechanisms:
Cytotoxic T lymphocytes (CTLs)
As the primary effector cells of adaptive antitumor immunity, CTLs recognize tumor-specific antigens presented by major histocompatibility complex (MHC) molecules through their T cell receptors (TCRs).
Natural killer (NK) Cells
NK cells mediate cytotoxicity through mechanisms similar to CTLs but also significantly participate in antibody-dependent cellular cytotoxicity (ADCC), in which they recognize antibody-opsonized target cells through their Fcγ receptors (CD16).
Macrophages
Certain activated macrophage populations can participate in tumor cell phagocytosis and mediate killing through the secretion of reactive oxygen and nitrogen species. Recent advances have also highlighted their role in T cell redirection through bispecific antibodies that simultaneously target tumor antigens and macrophage-activating receptors.
Core Services at Creative Biolabs
Creative Biolabs offers a comprehensive range of advanced services optimized for accuracy, throughput, and physiological relevance, surpassing standard plate-based readouts.
- Standardized Potency Assays: Creative Biolabs' standardized potency assays are designed to provide quantitative, reproducible, and clinically predictive data to support product characterization and batch release testing.
- Custom Assay Development: Custom solutions for complex scenarios such as solid tumor microenvironments, exhaustion models, and combination drug evaluation.
- Mechanism of Action Studies: In-depth analyses, including immune synapse characterization, calcium flux monitoring, and metabolic profiling during co-culture.
T Cell Mediated Tumor Cell Lysis Assays
As a cornerstone of adaptive anti-tumor immunity, T cell-mediated cytotoxicity requires specialized assessment platforms. At Creative Biolabs, our carefully designed T cell-mediated tumor cell lysis assays are designed to unravel the complex cascade of T cell recognition, activation, and lethal delivery.
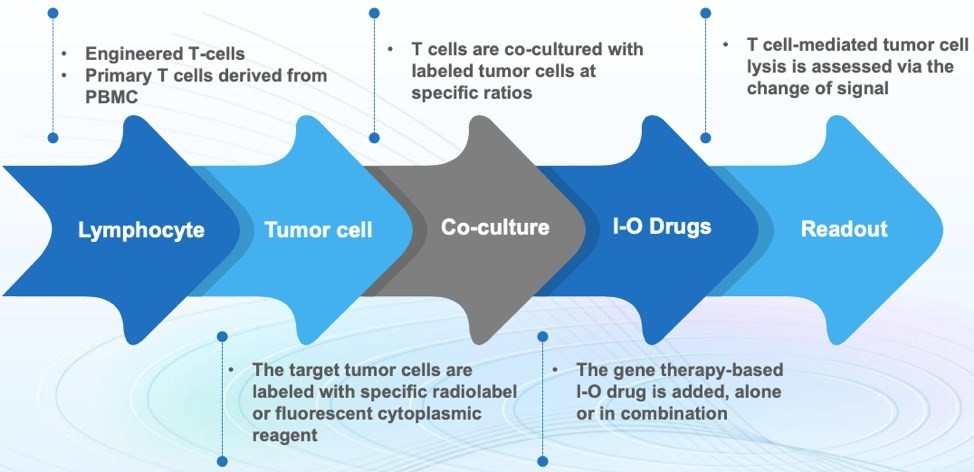 Figure 2. T Cell-mediated Tumor Lysis Assay.
Figure 2. T Cell-mediated Tumor Lysis Assay.
We have developed multiple assays to capture key stages of T cell killing:
- Immune synapse formation: Utilizing high-resolution live-cell imaging, we visualize the structured interface between the T cell receptor (TCR) and the tumor peptide-MHC complex.
- T cell activation and signaling: We quantify the intensity of intracellular signaling using calcium flux analysis and phosphorylation-specific flow cytometry.
- Cytotoxic granule polarization and exocytosis: Detecting CD107a surface exposure allows for direct measurement of degranulation and the release of perforin and granzymes.
- Induction of target cell apoptosis: We employ multiplexed approaches to confirm apoptosis through caspase-3/7 activation, Annexin V binding, and DNA fragmentation, distinguishing between perforin/granzyme and Fas/FasL pathways.
Methods of Immune Cell-mediated Tumor Lysis Assay
Currently, there are two commonly used approaches to validate the immune cell-mediated tumor cell lysis, namely the radiolabeling method and in vitro live-cell imaging assay. In briefly, the targeted tumor cells are labeled with specific radiolabel or fluorescent cytoplasmic reagent, and then recognized by lymphocytes. Through real-time monitoring the change of radiolabel or fluorescence intensity in targeted tumor cells, the immune cell-mediated tumor cell lysis can be measured. Of course, we also provide the ratio optimization of the co-culture lymphocytes and tumor cells to quantify cytolytic activities of lymphocytes against target cells. GTOnco™ services can be also designed to meet our clients' special needs to support their gene therapy-based I-O products development.
Our Collaborative Process
We collaborate with partners through a structured, scientifically rigorous workflow to ensure data delivery directly addresses research questions.
Consulting and Design
Detailed discussions on effector/target cell sources, drug specifications, and the determination of critical ratios and kinetic time points.
Cell Preparation and Quality Control (QC)
Stringent quality control of all cell components, including effector cell viability, phenotype, and functional readiness, is performed.
Experimental Execution
Optimized ICTLA protocols are implemented using state-of-the-art equipment, such as high-throughput flow cytometry (FACS).
Data Analysis
Raw and analytical data are provided, including curve fitting for EC50/IC50 calculations, statistical comparisons, and the generation of publication-ready figures and tables.
Tumor Lysis Assay Service Addressing Complex Biological Challenges
Overcoming Tumor Heterogeneity: We design assays targeting antigenically heterogeneous tumor populations to simulate and study antigen escape.
Mimulating the Immunosuppressive Tumor Microenvironment (TME)
We incorporate regulatory T cells (Tregs), M2 macrophages, or immunosuppressive cytokines to test T cell function under stress.
Assessing T Cell Exhaustion
Through repeated cycles of antigen stimulation, we simulate T cell exhaustion and evaluate the effects of interventions on restoring T cell function and lifespan.
Modeling Solid Tumors
We utilize 3D tumor spheroids and patient-derived organoids to assess T cell infiltration and killing within physiologically relevant structures.
Applications of Our Services
Our services cover the following areas in immunotherapy:
- CAR-T/NK cell development and optimization
- Bispecific antibody screening and lead candidate drug selection
- Immune checkpoint modulator evaluation
- Adoptive cell therapy potency testing and batch release
- Tumor resistance mechanism research
Results Delivery
- Comprehensive analytical output (dose-response curves, kinetic curves).
- Advanced data visualization through interactive dashboards.
- Statistical comparison and ranking of drug candidates.
- Access to complete raw data in multiple formats.
- Expert interpretation and strategic recommendations.
Our Advantages
Creative Biolabs' expertise translates directly into unparalleled data quality and utility.
Physiological Relevance
We prioritize 3D co-culture models (spheroids, organoids) over traditional 2D cell monolayers, providing a more tumor-like environment and better predicting in vivo efficacy.
Kinetic Resolution
Using label-free RTCA and state-of-the-art technologies, we enable precise quantification of the killing process, highly effective drugs and slower, less effective drugs.
Frequently Asked Questions
Q: How long does a complete tumor lysis assay typically take?
A: Our standard workflow takes 2-3 weeks. Complex studies involving primary cells or custom models may take 4-6 weeks. We offer expedited service for urgent requests.
Q: How do you address inter-donor variability in immune cell responses?
A: We typically use cells from multiple donors (usually 3-5) to capture biological variability. Our statistical models account for this, and we offer matched donor sets for specific studies.
Q: Can your T cell assays simulate T cell exhaustion?
A: Yes. We have established protocols for inducing and monitoring T cell exhaustion with repeated antigen stimulation, allowing for the evaluation of interventions designed to restore T cell function.
Q: What are the advantages of live imaging over flow cytometry for T cell assays?
A: Live imaging can provide kinetic data (e.g., time to killing, serial killing activity) that endpoint flow cytometry cannot capture. We often use the two complementary approaches: imaging for rich kinetic data and flow cytometry for high-throughput, multiparameter phenotypic analysis.
Q: What value does a GEO submission bring to my research?
A: Data uploaded to the Gene Expression Omnibus (GEO) database can significantly improve the visibility and reproducibility of research findings. High-quality ICTLA data, especially when combined with transcriptomic or proteomic data (e.g., changes in tumor antigen expression after treatment), can be extremely valuable to the broader scientific community, increasing citations and establishing your data as a public resource.
Connect with Us Anytime!
As a leading life science service provider, Creative Biolabs has successfully established advanced in vitro validation platform for our clients all over the world. our scientists will professionally perform the immune cell-mediated tumor lysis assay to verify the anti-tumor activity of I-O products. Please feel free to contact us and we will get back to you as soon as possible.
Reference
- Lupușoru G, Ailincăi I, Frățilă G, et al. Tumor lysis syndrome: an endless challenge in onco-nephrology. Biomedicines, 2022, 10(5): 1012. https://doi.org/10.3390/biomedicines10051012 (Distributed under Open Access license CC BY 4.0, without modification.)
