Introduction Published Data What We Can Offer? Workflow Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Are you facing challenges in protein expression and purification, or a need for highly specific complement regulators for your research or therapeutic development? Creative Biolabs' soluble complement regulators development service, specifically our C4-binding Protein (C4BP) Development Service, is designed to help you accelerate drug discovery and obtain high-quality recombinant proteins. Leveraging advanced recombinant DNA technology and innovative protein engineering techniques, we provide the precise C4BP constructs you need to streamline your project's success.
Contact our team to get an inquiry now!
Introduction
The complement system is a crucial part of the innate immune response, providing a first line of defense against pathogens. However, its powerful lytic and inflammatory capabilities must be tightly regulated to prevent damage to host tissues. C4b-binding protein (C4BP) is a key soluble regulator of the classical and lectin complement pathways. This large, multimeric glycoprotein consists of a variable number of identical α-chains and a unique β-chain. The most common human isoform is composed of seven α-chains and one β-chain, giving it a characteristic octopus-like structure. C4BP acts as a cofactor for the serine protease Factor I, mediating the proteolytic inactivation of C4b and C3b, which are central components of the complement cascade. By accelerating the decay of C3 convertases, C4BP effectively shuts down complement activation. Dysregulation or dysfunction of C4BP can contribute to various diseases, including autoimmune disorders and inflammatory conditions, highlighting its critical role in immune homeostasis.
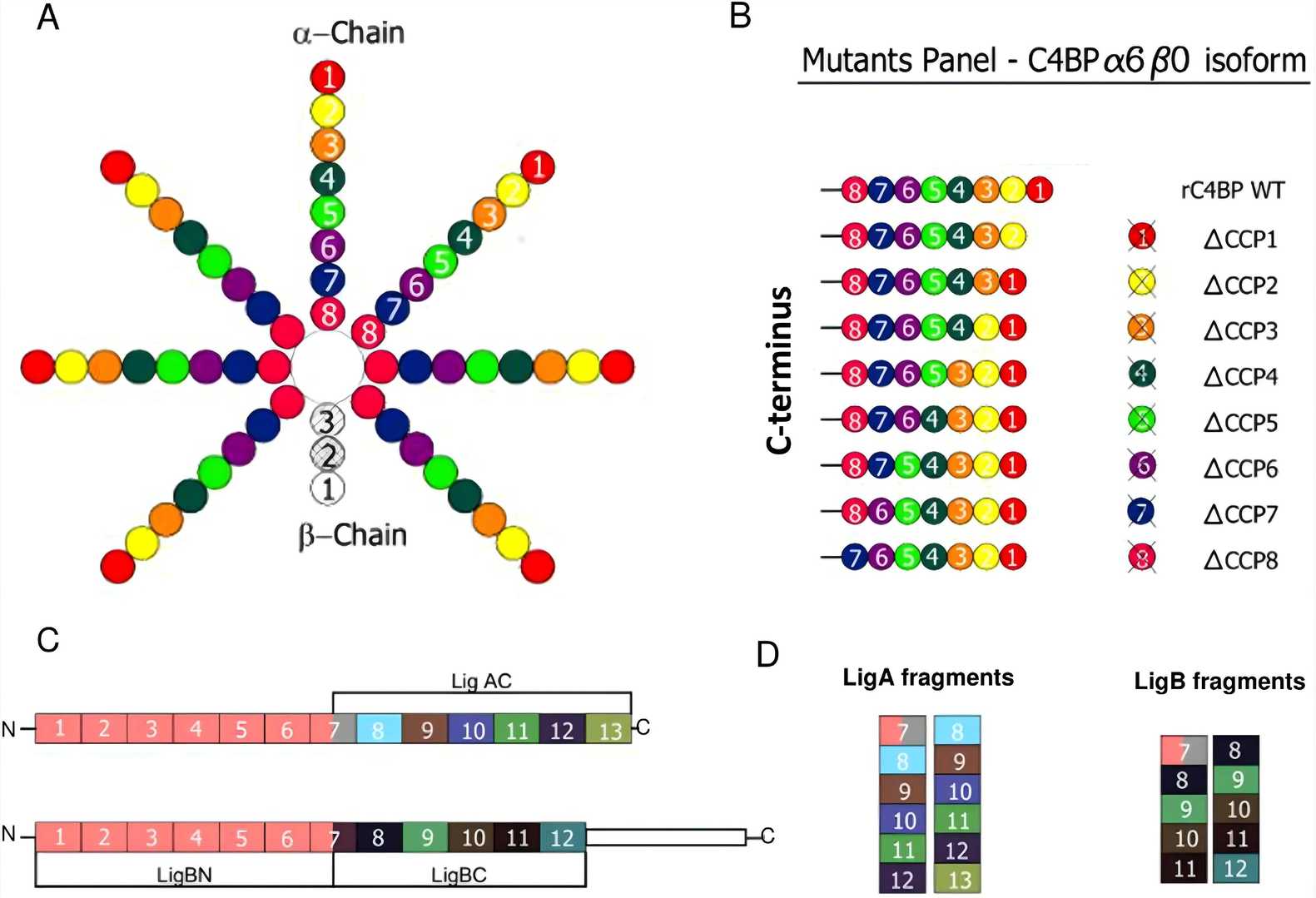 Fig.1 Structure of C4BP.1,3
Fig.1 Structure of C4BP.1,3
C4BP and Diseases
While C4BP is essential for maintaining immune balance, its dysregulation can have significant pathological consequences. An imbalance in C4BP's function can lead to uncontrolled complement activation, which contributes to a range of inflammatory and autoimmune disorders. Understanding the role of C4BP in these disease states is critical for developing new diagnostic tools and therapeutic interventions.
-
Complement-Related Pathologies: Uncontrolled complement activation can contribute to:
-
Inflammatory disorders such as rheumatoid arthritis, systemic lupus erythematosus, and neurodegenerative conditions.
-
Organ transplantation rejection.
-
Pathogen-mediated immune evasion, where microbes hijack C4BP to escape complement-mediated killing.
-
Diagnostic Marker: Beyond its role in complement regulation, C4BP has also been identified as a potential diagnostic marker. Published data indicate that elevated levels of C4BPA may be associated with right ventricular remodeling in patients with certain cardiovascular conditions.
-
Therapeutic Potential: Soluble complement regulators like C4BP represent a promising class of therapeutics. By introducing a modified or recombinant version of a natural inhibitor, it is possible to dampen excessive complement activation at a specific site of inflammation, offering a powerful strategy for treating diseases where complement overactivity is a primary driver of pathology.
Published Data
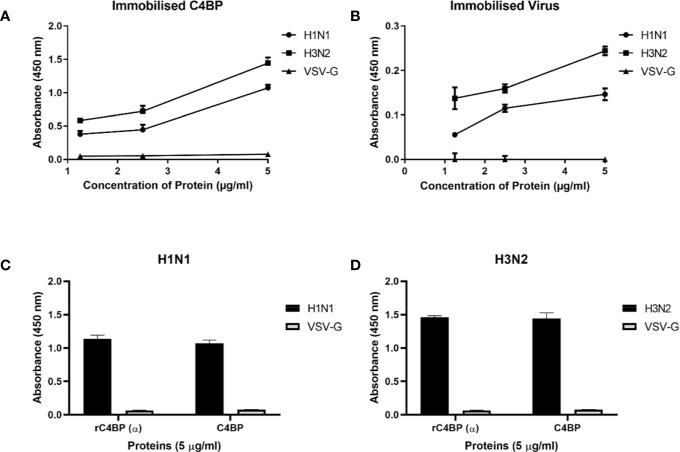 Fig.2 Binding interaction of immobilized C4BP to IAV subtypes.2,3
Fig.2 Binding interaction of immobilized C4BP to IAV subtypes.2,3
Recently published data explores the role of C4b-binding protein (C4BP) as an innate immune effector against the influenza A virus (IAV). The study investigated the direct interaction between recombinant human C4BP and IAV, specifically focusing on the viral surface protein, hemagglutinin (HA). Experimental findings demonstrated that C4BP binds to the IAV virion in a dose-dependent manner, and this binding is mediated by a specific interaction with the viral hemagglutinin protein. Further analysis showed that the binding occurs through the sialic acid-binding domain of HA. Importantly, the research revealed a direct antiviral effect, where C4BP was shown to inhibit IAV infection of host cells. The data suggest that this antiviral activity is independent of complement-mediated pathways, highlighting a novel and direct mechanism by which C4BP contributes to the innate immune response. The findings support the development of C4BP as a potential therapeutic agent for combating viral infections.
What We Can Offer?
To support your research from every angle, Creative Biolabs offers a comprehensive suite of products and services related to C4BP and the complement system.
-
Recombinant C4BP Proteins: We provide a catalog of high-quality recombinant C4BP proteins, including human and other species' isoforms.
-
Custom Protein Engineering: Design and development of novel C4BP constructs, fusion proteins, and truncated forms with enhanced properties.
-
Functional Assays: A range of validated assays to test C4BP's cofactor activity, decay-accelerating activity, and binding to C4b.
-
Antibody Development: Generation of highly specific monoclonal and polyclonal antibodies targeting C4BP for research and diagnostic applications.
Workflow
01
Gene Synthesis and Vector Construction: We synthesize the gene encoding your custom C4BP construct, optimizing it for expression in your chosen host system, and clone it into a high-efficiency expression vector.
02
Expression and Protein Engineering: The engineered vector is expressed in a suitable host system (e.g., mammalian cells). We can introduce specific mutations to enhance stability, modify binding properties, or create novel fusion proteins.
03
Protein Purification and Characterization: The C4BP protein is purified using a combination of techniques such as affinity chromatography, ion-exchange, and size-exclusion chromatography to ensure high purity.
04
Quality Control and Validation: The purified protein undergoes rigorous quality control, including SDS-PAGE, Western blot, and Mass Spectrometry, to confirm purity, identity, and molecular weight.
05
Functional Analysis: We perform functional assays, such as binding assays and complement inhibition tests, to validate the biological activity of the C4BP construct.
Why Choose Us?
Choosing the right partner for your soluble complement regulator project is crucial. At Creative Biolabs, our expertise in C4BP development is underpinned by a deep understanding of protein science and a commitment to delivering superior results.
-
Unmatched Expertise and Experience: Our team has over two decades of experience in protein engineering, expression, and purification, ensuring precision and efficiency for complex projects.
-
Customization and Flexibility: Our services are fully customizable to your specific needs, from designing a novel C4BP fusion protein to optimizing expression for challenging constructs.
-
Rigorous Quality Control: Every protein undergoes a multi-step quality assurance process, guaranteeing high purity, correct molecular weight, and confirmed biological activity.
-
Data-Driven Results: We provide a comprehensive data package with every project, including raw data and detailed analysis to ensure full transparency.
Obtain the Creative Biolabs Advantage – Seek a Quote Today
FAQs
Q: Can a recombinant C4BP protein be used to replace a natural C4BP protein for research purposes?
A: Yes, a well-engineered recombinant C4BP can serve as an excellent research tool. Recombinant proteins offer the advantage of high purity, lot-to-lot consistency, and can be engineered with specific tags or mutations to better suit your experimental design, which is often difficult to achieve with proteins purified from serum.
Q: How does C4BP development relate to therapeutic applications?
A: Developing a therapeutic C4BP protein can be beneficial for treating conditions where overactive complement activation is a problem. By designing a C4BP that is more potent or stable, it may be possible to create a targeted therapy that down-regulates the classical or lectin pathways, offering a novel approach to managing inflammatory or autoimmune diseases.
Q: What are the main challenges in developing a soluble complement regulator?
A: A key challenge is ensuring the protein maintains its correct multimeric structure and biological activity. C4BP, with its complex multi-chain structure, requires specialized expression systems and careful purification to produce a functional protein. This is where partnering with an experienced provider becomes invaluable.
Q: How can I be sure that the C4BP protein I receive will be functional in my specific assay?
A: We understand the need for functional reliability. Our quality control includes a range of assays, from general binding to specific decay-acceleration tests, to confirm biological activity. We can also work with you to customize a functional assay specific to your application to provide an extra layer of confidence.
Q: Why would I choose to use a C4BP construct instead of other complement inhibitors?
A: C4BP provides a unique advantage by specifically targeting the classical and lectin pathways. This selectivity can be beneficial in applications where you want to leave the alternative pathway intact, providing a more nuanced approach to complement inhibition compared to broader-acting inhibitors. The choice depends on the specific biological question you are trying to answer.
Creative Biolabs is a leading provider in the field of soluble complement regulators, with a specific focus on C4-binding protein development. We offer a full-service experience, from initial construct design to final protein delivery, ensuring your project advances efficiently. Our expertise and commitment to quality make us the ideal partner for your C4BP research and development needs.
Featured Services
Feature Products
References
-
Breda, Leandro C D et al. "Fine Mapping of the Interaction between C4b-Binding Protein and Outer Membrane Proteins LigA and LigB of Pathogenic Leptospira interrogans." PLoS Neglected Tropical Diseases vol. 9,10 e0004192. 30 Oct. 2015, https://doi.org/10.1371/journal.pntd.0004192
-
Varghese, Praveen M et al. "C4b Binding Protein Acts as an Innate Immune Effector Against Influenza A Virus." Frontiers in Immunology vol. 11 585361. 8 Jan. 2021, https://doi.org/10.3389/fimmu.2020.585361. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig.1 Structure of C4BP.1,3
Fig.1 Structure of C4BP.1,3
 Fig.2 Binding interaction of immobilized C4BP to IAV subtypes.2,3
Fig.2 Binding interaction of immobilized C4BP to IAV subtypes.2,3