Product List Background C3 Convertase Functional Service
Background
Activation of complement is very important for protection against microbial infections, but over-activation of complement leads to host tissue damage. The complement system is activated in two ways: (1) specific recognition of target cells in the classical and lectin pathways and (2) spontaneously due to the inherent instability of complement component C3 in the alternative (AP) pathways. These pathways converge in the generation of C3 convertases, which cleave C3 into the small anaphylatoxin C3a and the large C3b, which may bind to target surfaces. In the amplification loop of the AP pathway, pro-enzyme factor B (FB) binds to surface-bound C3b and is cleaved by factor D causing an active convertase complex that is made up of C3b and the non-covalently bound protease fragment Bb (denoted C3bBb).
C3 convertases are produced in the classical and lectin pathways via C4 and C2, which are homologs of C3 and FB respectively. It is worth noting that both C3 convertases (C3bBb and C4b2a) are active only towards their natural substrate C3, and with limited activity towards the homolog C5. Besides, in the terminal complement pathway, the substrate specificity is switched from C3 to C5 after the association of one or more C3b molecules to the C3bBb or C4b2a complexes. In addition, the complement activity can be regulated via convertase assembly and disassembly, which is controlled by complement regulators.
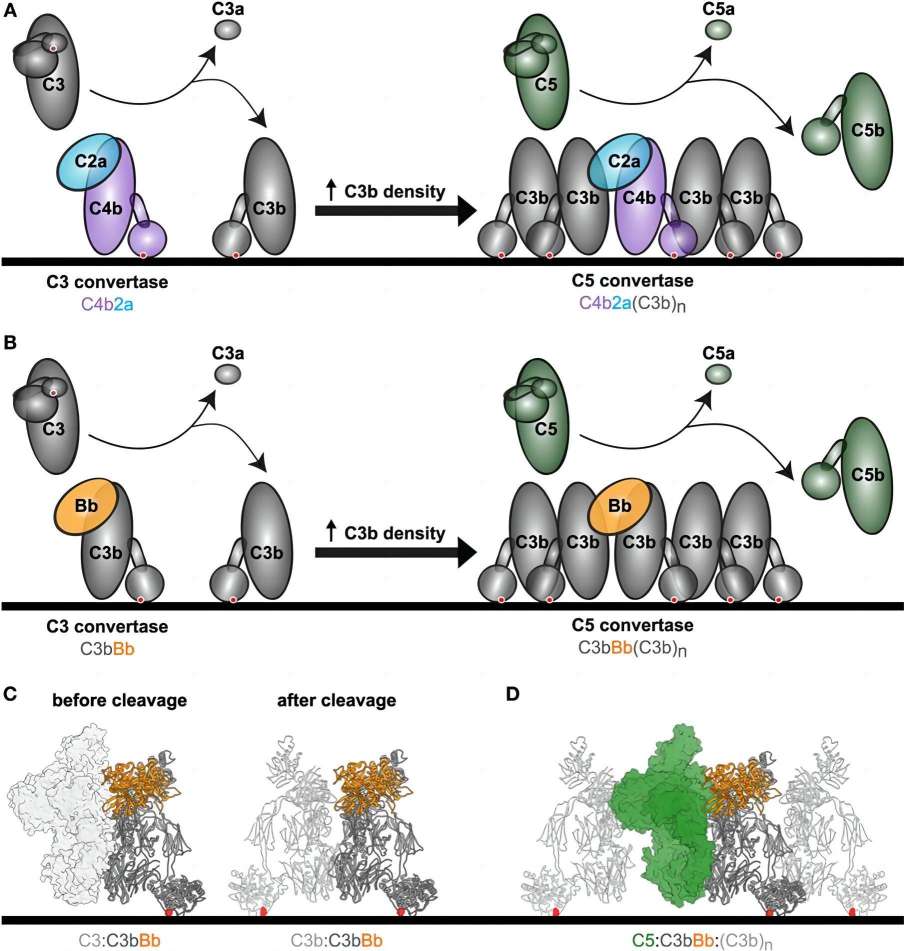 Fig.1 C3 processing facilitated by complement convertases.1, 3
Fig.1 C3 processing facilitated by complement convertases.1, 3
C3 Convertase Functional Service
Creative Biolabs presents an extensive range of products centered around C3 convertase, including ELISA kits for detecting C3 convertase and inhibitors targeting this enzyme. These carefully crafted reagents are crucial in advancing research endeavors focused on developing therapeutic approaches for various diseases.
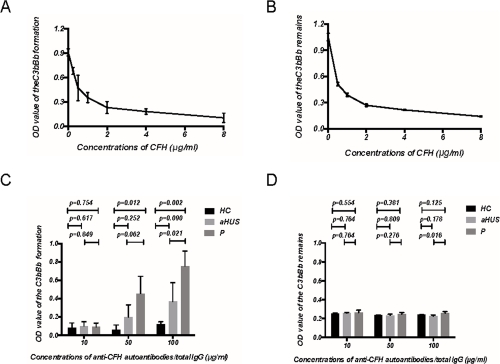 Fig.2 Assay for formation and decay of C3 convertase.2, 3
Fig.2 Assay for formation and decay of C3 convertase.2, 3
C3 glomerulonephritis (C3GN) is identified as an uncommon condition originating from the disruption of the complement alternative pathway (CAP), which may arise from genetic causes or be linked to monoclonal gammopathy of renal significance (MGRS). Researchers encountered a patient with MGRS-associated C3GN, characterized by reduced serum C3 levels, haematuria, and nephrotic syndrome, facing a rapid decline in kidney function within 10 months. Investigations revealed that purified anti-CFH autoantibodies from the patient’s plasma were monoclonal IgGλ, obstructing CFH binding to C3b and enhancing C3 convertase formation. Using ELISA, researchers assessed CFH’s capability to modulate the alternative pathway C3 convertase dynamics.
Creative Biolabs provides a comprehensive range of C3 convertase-focused functional services, offering detailed interaction analyses and an array of specialized evaluations. These precisely tailored services are crafted to aid researchers in advancing the frontiers of their scientific research and clinical initiatives.
References
-
Zwarthoff, Seline A., et al. "Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models." Frontiers in immunology 9 (2018): 1691.
-
Li, Lin-Lin, et al. "Monoclonal immunoglobulin mediates complement activation in monoclonal gammopathy associated-C3 glomerulonephritis." BMC nephrology 20 (2019): 1-10.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 C3 processing facilitated by complement convertases.1, 3
Fig.1 C3 processing facilitated by complement convertases.1, 3
 Fig.2 Assay for formation and decay of C3 convertase.2, 3
Fig.2 Assay for formation and decay of C3 convertase.2, 3
