Aromatic l-amino acid decarboxylase deficiency
Adeno-associated viral vectors (AAVs) are the ideal therapeutic gene delivery system for various neurological diseases' treatment. Empowered by our advanced technology platforms and experienced technical personnel, Creative Biolabs offers a comprehensive set of AAV design and engineering services to assist our clients in gene therapy and provide a guideline for project progression.
Introduction to Aromatic L-amino Acid Decarboxylase Deficiency
Aromatic L-amino acid decarboxylase (AADC), also referred to as 5-hydroxytryptophan decarboxylase and DOPA decarboxylase, is a pyridoxine-dependent enzyme encoded by the human DDC gene. In humans, AADC is mainly responsible for the respective conversion or decarboxylation of L-DOPA and 5-hydroxytryptophan to dopamine and serotonin, both of which are important neurotransmitters in brain signal transduction. Mutation in the DDC gene causes AADC deficiency, an extremely rare, autosomal recessive neurometabolic disorder. This is a severe combined deficiency as the reduced activity of AADC results in insufficient production of dopamine, serotonin, norepinephrine, and epinephrine, contributing to the developmental delay, abnormal movements (i.e., Parkinson's disease), and autonomic dysfunction in AADC deficiency patients.
Present management and treatments of AADC deficiency are mostly symptomatic aiming to relieve the signs and symptoms of the disease. Various medications are administrated to compensate for the correspondingly absent neurotransmitters. The most potential treatment of AADC deficiency under trails is to correct the mutated genes or deliver normal human DDC genes into the neurons to express enough AADC.
AAV Vector-Based Gene Therapy for Aromatic L-amino Acid Decarboxylase Deficiency
AAV has emerged as a perfect vehicle for gene therapy of AADC deficiency owing to its neuro-tropism, low-pathogenicity, and infectivity of non-dividing cells. Normal human DDC genes are transferred by the AAV vector directly across the blood-brain barrier into non-degenerating striatal neurons. Gene therapy for AADC deficiency treatment under preclinical studies has indicated that the AAV transferred DDC genes can be expressed for more than 7 years, which greatly improves therapeutic effects while reduces the frequency and cost of conventional medication.
AAV vector-based gene therapy for AADC deficiency also provides promise for the treatment of Parkinson's disease, a neurodegenerative disorder characterized by dopamine insufficiency. Several phases 1/2 clinical studies have demonstrated that AAV type 2 mediated gene therapy by delivery of human DDC gene into the putamen of Parkinson's disease patients is of great efficacy, safety, and tolerability.
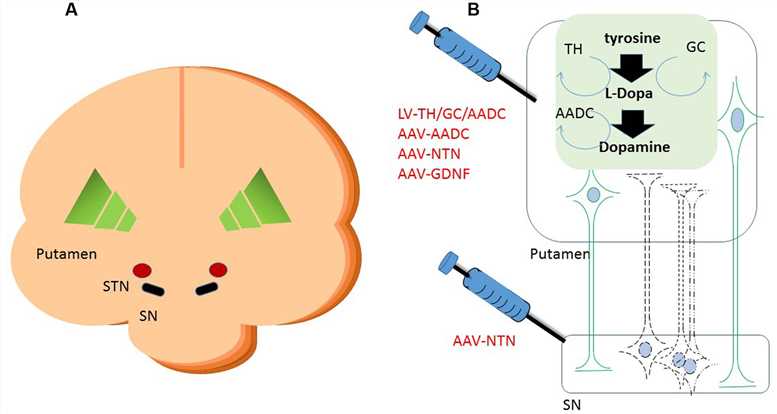 Figure 1. A schematic representation of gene therapeutic approaches to the putamen. (Blits, 2016)
Figure 1. A schematic representation of gene therapeutic approaches to the putamen. (Blits, 2016)
Features
- A number of AAV serotype products are available
- A full set of assays for measuring the potency and safety of gene therapy
- Advanced gene therapy technology platform
- Highly customized service according to different research demands
As a biotechnology company keeping pace with times, Creative Biolabs provides customized AAV vector design services for the gene therapy of AADC deficiency and Parkinson's disease. Please feel free to contact us and our experienced technicians will give you the most detailed answers to your questions.
Reference
- Blits, B.; Petry, H. (2016). Perspective on the Road toward Gene Therapy for Parkinson's Disease. Frontiers in Neuroanatomy. 10: 128. Distributed under Open Access license CC BY 4.0, without modification.
