AAV Vector Design Service for Amyotrophic Lateral Sclerosis (ALS)
Introduction
Amyotrophic lateral sclerosis (ALS) is a complex neurological disorder with long drug development cycles and challenges in effective therapy development. Our custom rAAV design service accelerates ALS drug discovery via advanced recombinant DNA tech and high-throughput screening, offering a data-driven path from target gene identification to optimized, ready-to-use vectors. It solves hurdles like inefficient transduction, off-target effects, and poor expression in motor neurons/muscle, delivering high-quality, customized vectors for impactful preclinical results.
Discover How We Can Help - Request a Consultation
Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) is a devastating neurodegenerative disease, with progressive motor neuron degeneration causing severe muscle atrophy and death. As a multifactorial disorder linked to over 50 genes, effective therapies are hard to develop. Yet research breakthroughs highlight gene therapy's potential. Our platform leverages this knowledge, focusing on diverse therapeutic approaches and genetic targets to offer credible, robust research solutions.
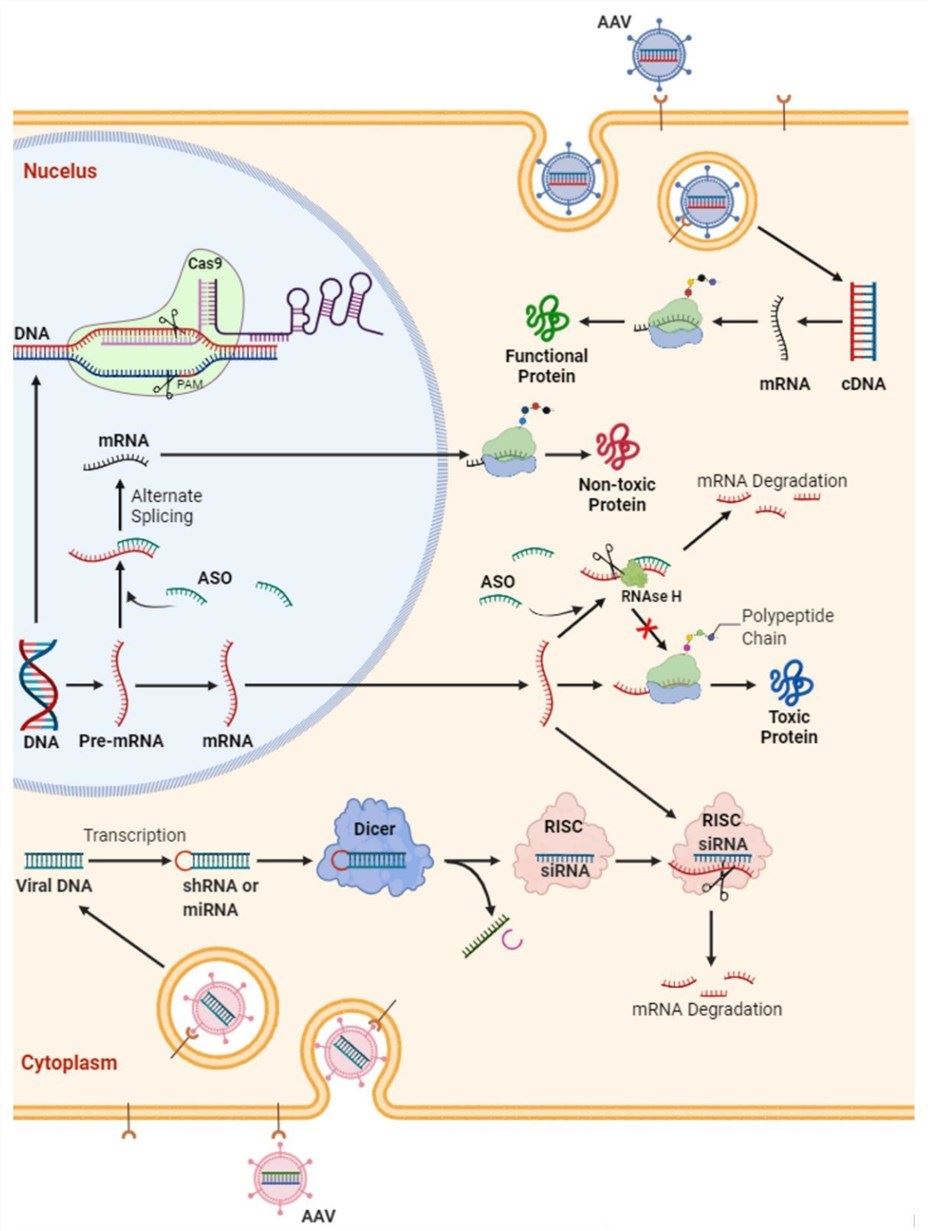 Fig.1 Schematic diagram of potential gene therapy strategies for amyotrophic lateral sclerosis. Delivering genes through adeno-associated virus vectors is another option for functional substitution of deleted genes.1
Fig.1 Schematic diagram of potential gene therapy strategies for amyotrophic lateral sclerosis. Delivering genes through adeno-associated virus vectors is another option for functional substitution of deleted genes.1
ALS pathogenesis involves the progressive loss of motor neurons in the brain and spinal cord, which in turn leads to the dismantling of the neuromuscular junction (NMJ) and severe muscle atrophy. While common treatments such as Riluzole and Edaravone can modestly slow disease progression, there is no cure. Gene therapy offers a promising new approach by targeting the underlying genetic and molecular pathways of the disease.
ALS gene therapy focuses on restoring gene function, silencing toxic proteins, or delivering neuroprotective factors, with key targets including SOD1, C9orf72, FUS, and TARDBP mutations—our expertise enables specific therapy design.
AAVs are preferred for neurological gene delivery. A recent study used AAV to deliver the NRIP gene (critical for sarcomere integrity and neuromuscular junction stability). Intramuscular AAV-NRIP reached spinal cord motor neurons via retrograde trafficking, improving motor function, boosting motor neuron survival, and reducing muscle atrophy in preclinical models, showing potential to address ALS symptoms and root causes.
Workflow
-
Required Starting Materials:
To initiate a project, clients typically provide detailed information on their desired therapeutic target, such as the full-length or partial DNA sequence of the gene of interest. Additionally, providing your specific research objectives and desired animal models is crucial to optimizing vector design for your specific needs. -
Key Steps Involved
- Target and Serotype Selection: Collaborate with you to select suitable AAV serotypes based on target cells (e.g., motor neurons, muscle cells) and tropism, ensuring maximum transduction efficiency and minimal off-target delivery.
- Vector Design and Construction: Design/construct rAAV vector plasmids with your target gene, tissue-specific promoters, and regulatory elements, using advanced molecular cloning and DNA sequencing for flawless constructs.
- Virus Packaging and Purification: Package plasmids into chosen AAV capsids via proprietary high-yield platforms, then purify viral particles to high purity/titer, removing contaminants that disrupt research.
- Quality Control and Validation: Conduct rigorous QC on each AAV batch (titer, purity, functionality) and perform in vitro/in vivo validation to confirm target cell transduction and desired gene expression.
- Final Delivery: Ship validated AAV vectors with a comprehensive technical report (project parameters, QC data, recommended usage protocols).
- Final Deliverables: Upon project completion, you will receive the purified, ready-to-use rAAV vector. This is accompanied by a detailed Certificate of Analysis containing the vector's titer and purity data, as well as a comprehensive Project Report outlining the methodology, validation results, and recommended protocols for your research.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 12 weeks, depending on the complexity of the gene target and the specific validation requirements of your project.
What we can offer
A seamless, one-stop solution from gene to vector
Our service manages every step of the process, from initial gene sequence optimization to the final, validated product, freeing you to focus on your research.
Tailored process development for maximum yield
We offer optimized upstream and downstream development, including custom culture conditions and scalable processes to maximize your vector yield.
Precision-engineered vectors for enhanced performance
We optimize gene codon usage and utilize the latest vector design techniques to ensure your gene is expressed efficiently in target cells.
Robust quality assurance through QbD and PAT
Our well-established quality system employs a Quality-by-Design approach and process analytical techniques to guarantee product consistency and reliability.
Certified quality and safety
We adhere to the basic principles of Good Manufacturing Practice (GMP) and implement strict aseptic verification to ensure the highest standards of quality control for your preclinical materials.
Expert consultation at every step
Our team provides comprehensive guidance on serotype selection, promoters, and vector modifications to create a fully customized solution that meets your project's unique requirements.
Experience the Creative Biolabs Advantage - Get a Quote Today
Case Study
| Western Blot | |
|---|---|
|
|
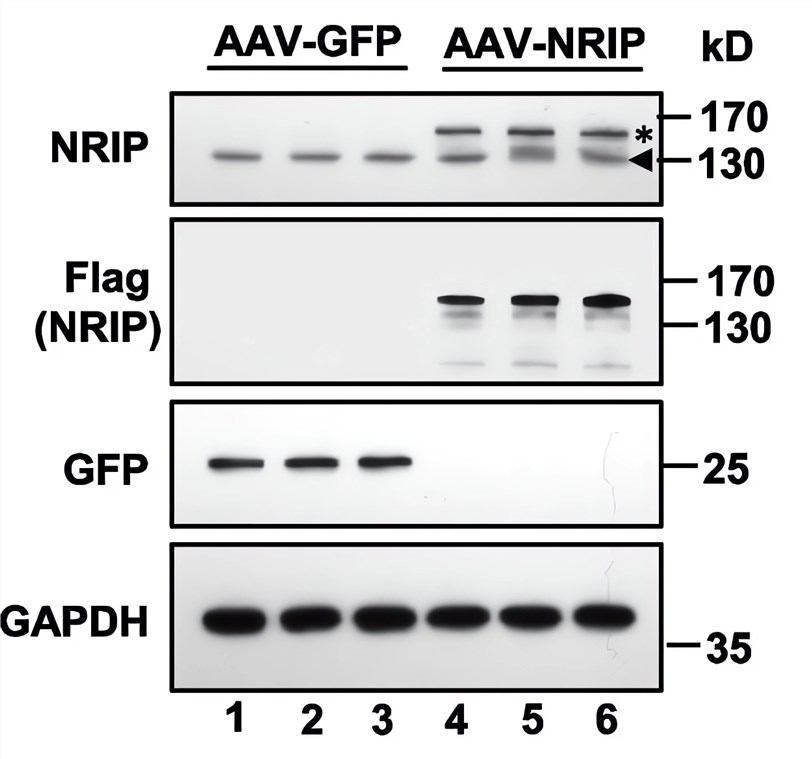
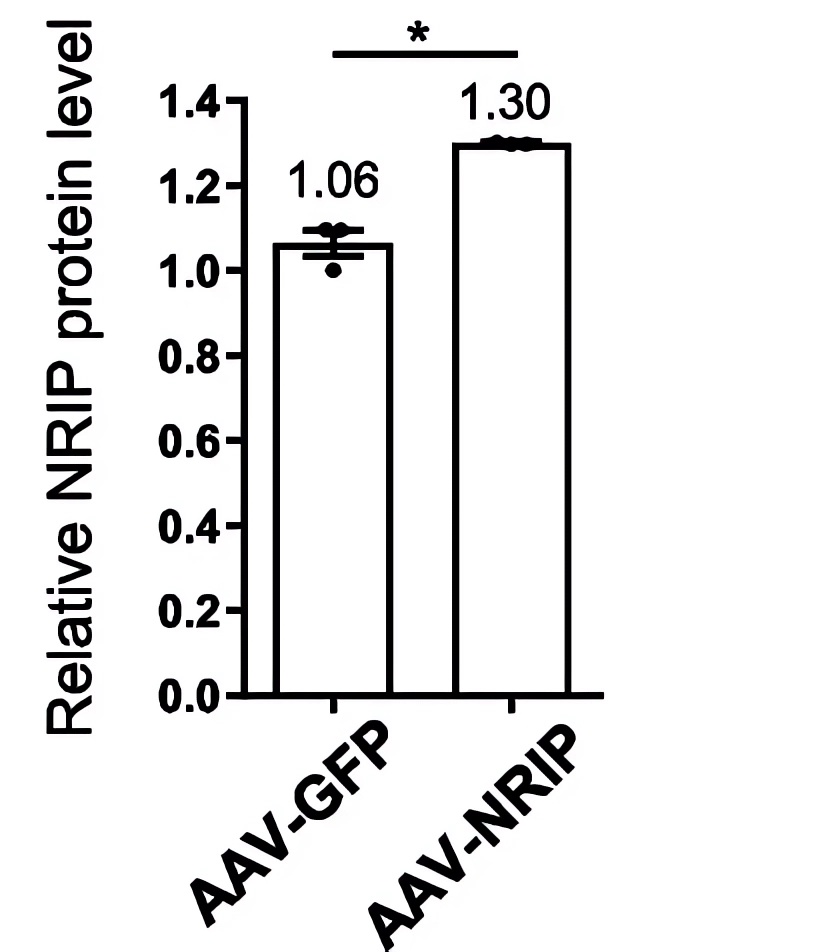
| Fig.2 The AAV encoding NRIP (AAV-NRIP) was constructed, and the expression of NRIP protein was detected by western blot experiment (AAV-GFP was used as the control group).2 | Fig.3 The expression level of NRIP was statistically analyzed.2 |
| Immunofluorescence | Analysis of Compound Muscle Action Potentials |
|
|
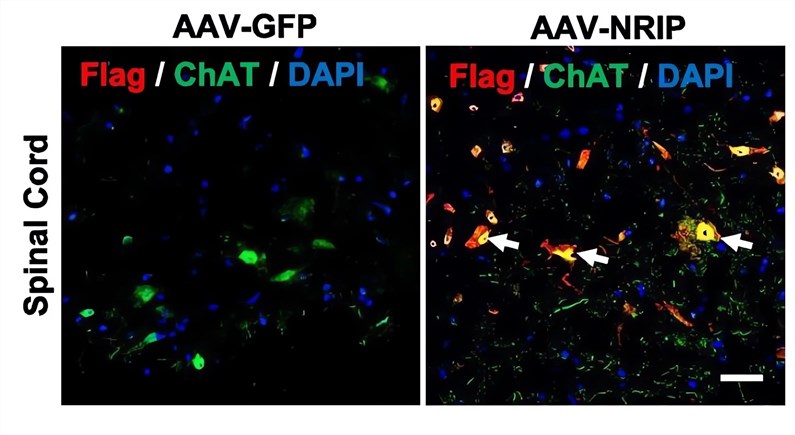
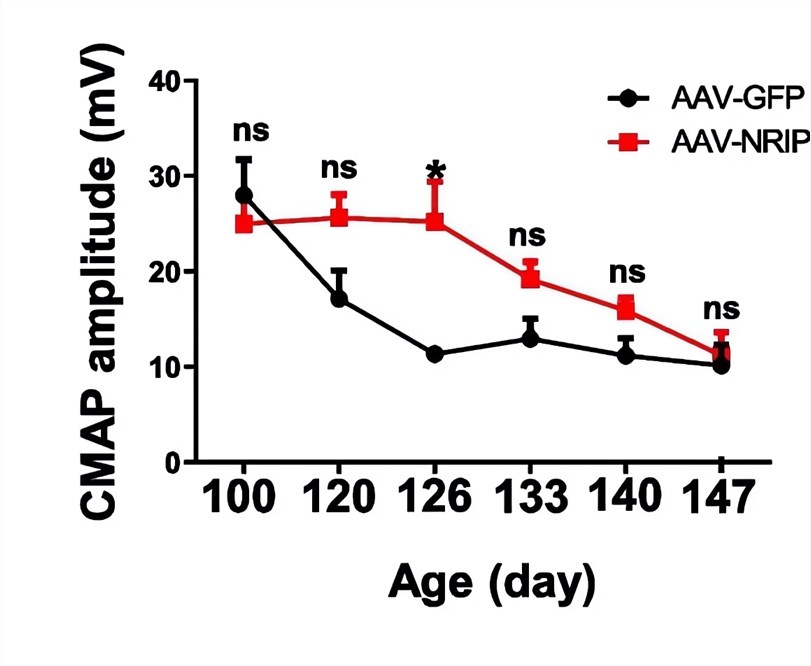
| Fig.4 The expression and localization of the NRIP protein were detected by immunofluorescence assay.2 | Fig.5 The amplitude of CMAP in ALS model mice treated with AAV-NRIP increased relatively.2 |
| Locomotor Activity | |
|
|
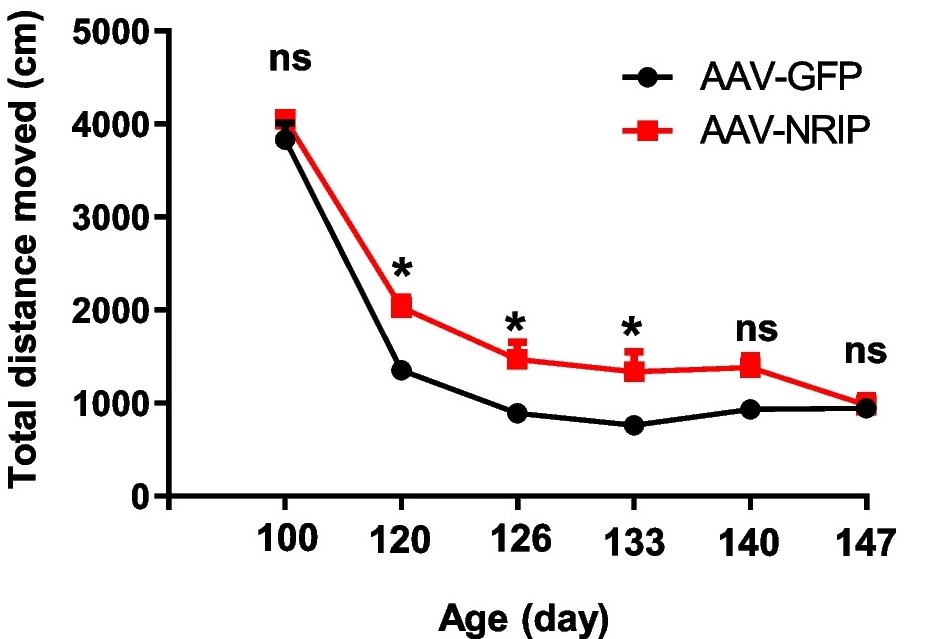
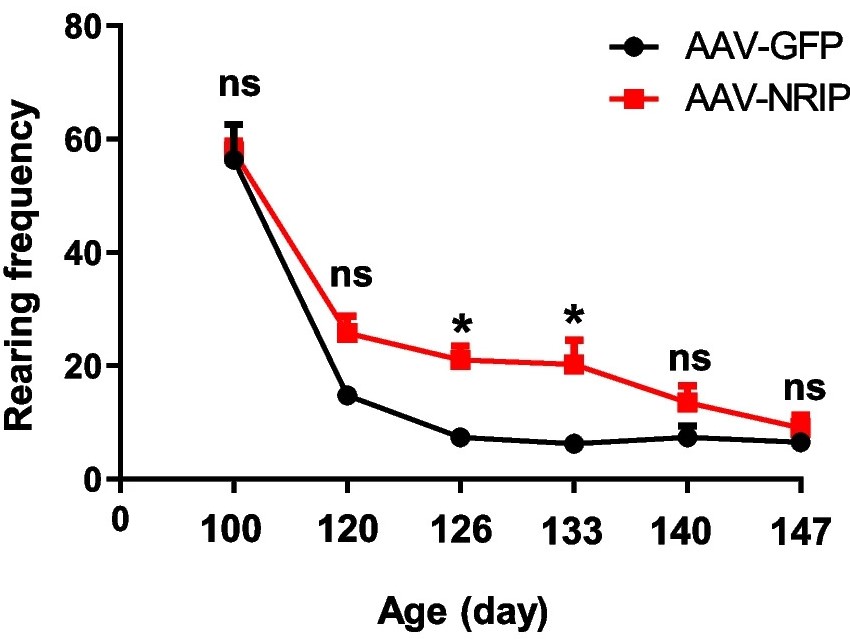
| Fig.6 The total movement distance of ALS disease model mice treated with AAV-GFP or AAV-NRIP.2 | Fig.7 The rearing frequency of ALS disease model mice treated with AAV-GFP or AAV-NRIP.2 |
Customer Reviews

FAQs
How do you select the most suitable AAV serotype for my project?
We have extensive experience with a broad range of AAV serotypes and their tropisms. We carefully analyze your project's specific goals, including your target tissue, desired expression level, and species, to recommend the optimal serotype that maximizes transduction efficiency and minimizes off-target effects. We also offer options for AAV capsid engineering to further enhance vector performance.
Can your service be used for genes other than SOD1 or C9orf72?
Absolutely. While we have deep expertise with common ALS targets, our platform is fully customizable to accommodate a wide variety of genes. Whether you're working with a new or less-studied gene like NRIP or an entirely different therapeutic target, our workflow is adaptable to your specific needs. Simply provide us with your gene of interest, and we can discuss the best approach for its delivery.
How do you ensure the quality and purity of the AAV vectors?
We employ a rigorous, multi-stage quality control process. Every batch is tested for total viral particles, infectious units, and purity to ensure it meets our strict standards. We use techniques such as qPCR for titer determination and SDS-PAGE for purity assessment. This ensures that the vectors you receive are consistent, reliable, and ready for your most critical experiments.
What kind of validation data can I expect to receive with my vector?
In addition to standard quality control, we provide a detailed project report that includes key validation data. This can include information on the vector's expression in a proxy cell line and a detailed protocol for effective delivery in your specific animal model. Our goal is to provide you with all the information you need to confidently and successfully conduct your experiments.
For more info on our custom rAAV design service for ALS and to discuss your project needs, reach out to our expert team. We're committed to accelerating your research and helping you achieve therapeutic goals.
Contact Our Team for More Information and to Discuss Your Project
References
- Fang, Ton, et al. "Gene therapy in amyotrophic lateral sclerosis." Cells 11.13 (2022): 2066. https://doi.org/10.3390/cells11132066. Distributed under Open Access license CC BY 4.0, without modification.
- Chen, Hsin-Hsiung, et al. "AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice." Skeletal Muscle 14.1 (2024): 17. https://doi.org/10.1186/s13395-024-00349-z. Distributed under Open Access license CC BY 4.0, figures were cropped.
