AAV Vector Design Service for Huntington's Disease (HD)
Introduction
Huntington's Disease (HD) is a devastating neurodegenerative disorder. Creative Biolabs' custom AAV solutions accelerate HD gene therapy research, addressing challenges like specific deep brain delivery and low transduction efficiency via advanced capsid engineering and proprietary screening. We provide targeted solutions: potent rAAV vectors with custom capsids, validation data, and reports, empowering progress with optimized gene delivery systems.
Discover How We Can Help - Request a Consultation
Huntington's Disease (HD)
Pathogenesis
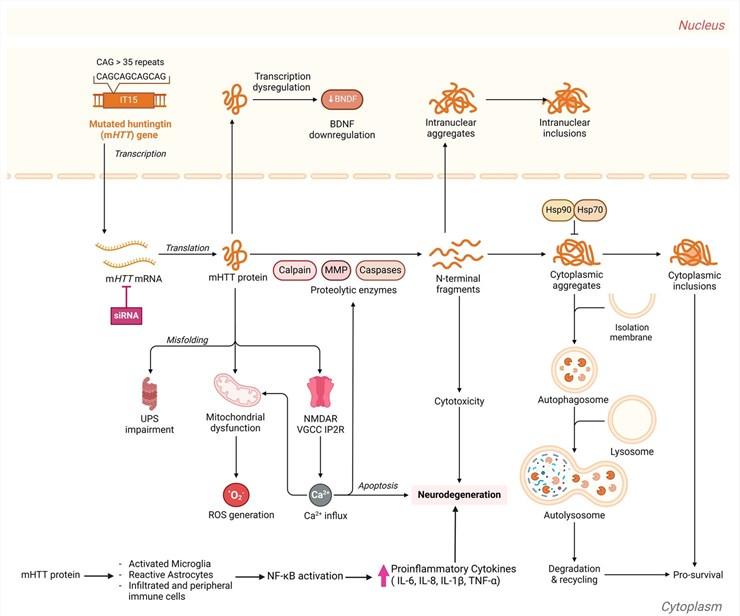 Fig.1 The complex molecular pathways and mechanisms of action of Huntington's disease.1
Fig.1 The complex molecular pathways and mechanisms of action of Huntington's disease.1
The pathogenesis of Huntington's Disease is rooted in the aggregation of the mutated huntingtin protein, which disrupts neuronal function and leads to the characteristic motor, cognitive, and psychiatric symptoms. Current common treatments for HD are largely symptomatic, focusing on managing the disease's effects rather than addressing the root cause. For example, tetrabenazine is used to treat chorea (involuntary movements).
Therapeutic Targets
Therapeutic targets for gene therapy in HD primarily focus on reducing the expression of the mutant HTT gene. This can be achieved through RNA interference (RNAi), which uses small interfering RNA (siRNA) or microRNA (miRNA) to trigger the degradation of the messenger RNA (mRNA) from the mutated gene, or through more permanent solutions, which can precisely edit the gene.
AAV-Based Treatments
AAV-based treatments lead in CNS therapeutic delivery, but widespread, potent transduction in deep brain structures (e.g., striatum) is a hurdle. AAV-DB-3, a novel capsid with superior delivery/transduction in these regions, marks a breakthrough for effective gene therapies for HD and other basal ganglia disorders.
Workflow
-
Required Starting Materials: To initiate your project, we typically require:
- Gene of Interest Information: The target gene sequence for silencing or for editing.
- Target Cell/Tissue Type: The specific neurons or brain regions you wish to target, such as medium spiny neurons in the striatum.
- Desired Expression Level: Information on the required therapeutic gene expression level or the level of HTT gene silencing needed.
-
Key Steps Involved:
- Discovery & Design: Start with an initial consultation to grasp project goals. Design custom rAAV capsids or screen proprietary libraries for optimized ones based on target/tropism, involving bioinformatic analysis and rational design.
- Vector Construction: Clone therapeutic payloads (e.g., silencing RNA cassettes) into optimized AAV backbones, ensuring high-fidelity construction and minimal off-target effects.
- Production & Purification: Produce vectors at high titer via proprietary large-scale processes, then purify to high standards to minimize impurities/endotoxins, critical for in vivo studies.
- In Vitro Validation: Test purified vectors in relevant cell lines (e.g., human iPSC-derived neurons) to assess transduction efficiency, specificity, and efficacy, providing initial proof of concept.
- In Vivo Validation: Deliver vectors to animal models (e.g., mice, non-human primates) to evaluate potency, distribution, and safety, confirming ability to cross barriers and reach deep brain structures.
-
Final Deliverables: Upon project completion, you will receive:
- Final Vector Product: High-titer, research-grade rAAV vector with the validated, custom-designed capsid.
- Detailed Report: A comprehensive report including all vector specifications, quality control data, and detailed results from in vitro and in vivo validation studies (e.g., transduction efficiency, biodistribution maps).
- Raw Data: All raw data and analysis files, allowing for independent verification and further research.
- Estimated Timeframe: The typical timeframe for this service ranges from 8 to 16 weeks, depending on the complexity of the capsid design, the therapeutic payload, and the scope of the in vivo validation.
What we can offer
Our Advantage
Customized rAAV Capsid Design
We don't just provide off-the-shelf solutions. Our expertise allows us to custom-engineer rAAV capsids with enhanced tropism for specific deep brain regions, ensuring precise delivery of your therapeutic payload.
High-Fidelity Screening Platforms
Our proprietary screening platforms enable the rapid and efficient discovery of novel AAV variants that exhibit unparalleled potency and reduce the potential for off-target effects.
Comprehensive Validation
We provide a complete validation package, including rigorous in vitro and in vivo studies in relevant models to confirm the efficacy, biodistribution, and safety of your custom vector.
Optimized for CNS Delivery
We specialize in overcoming the unique challenges of delivering gene therapies to the central nervous system, providing a solution specifically designed for complex diseases like Huntington's.
>Experience the Creative Biolabs Advantage - Get a Quote Today
Case Study
| The Expression Status of the Target miRNA | |
|---|---|
|
|
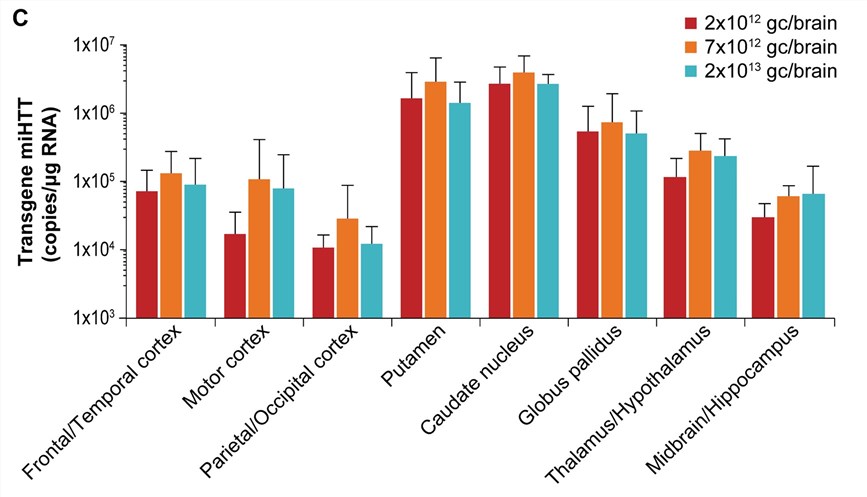
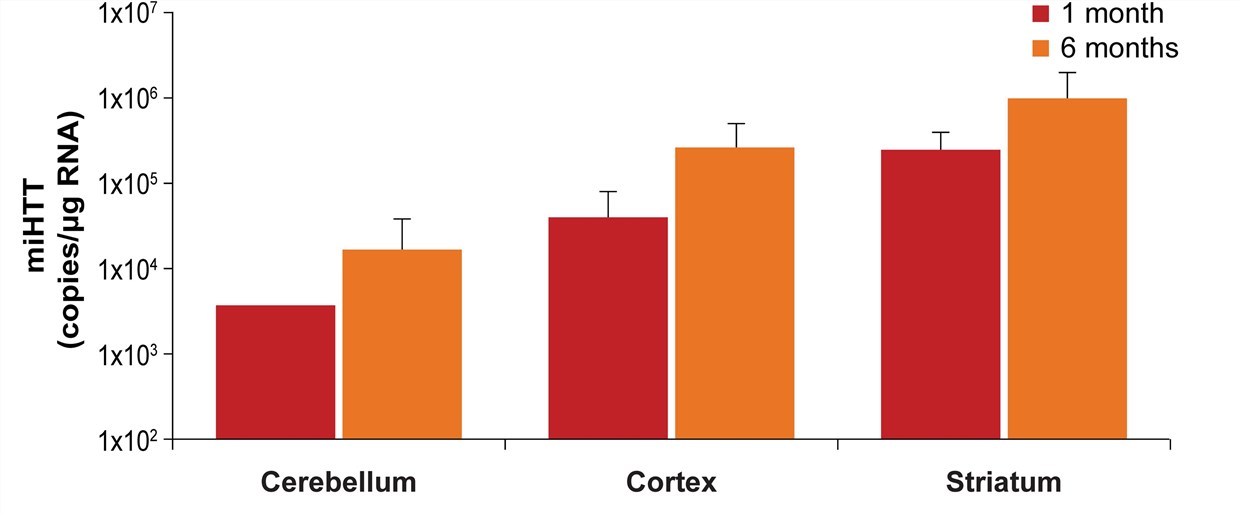
| Fig.2 The content of AAV-miHTT in various brain regions after injection.2 | Fig.3 The expression of mi HTT was at the highest level in the striatum 6 months after injection.2 |
| Brain Punch Analyses | Shedding of Vector DNA |
|
|
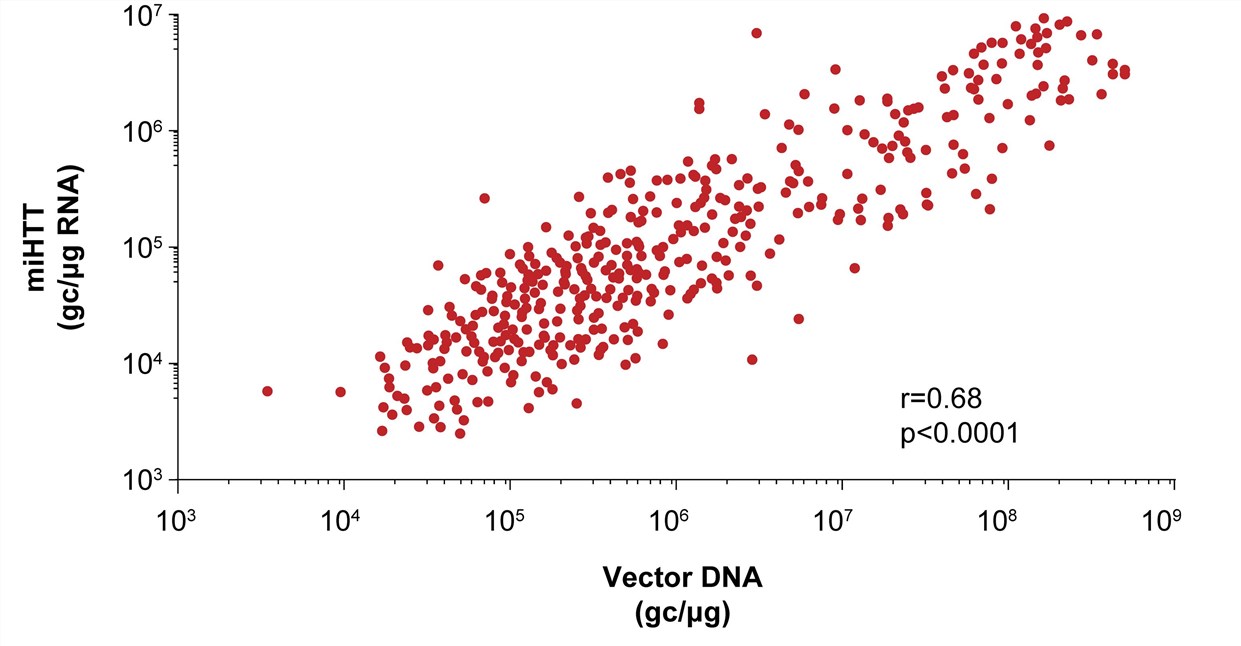
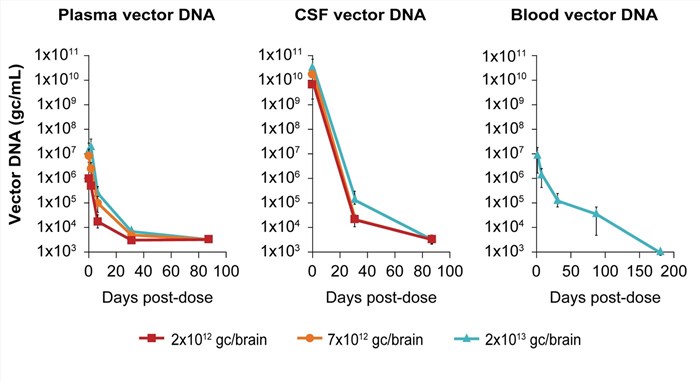
| Fig.4 The content of mi HTT shows a positive correlation trend with the dosage of injected AAV drugs.2 | Fig.5 The residual condition after the injection of AAV-miHTT drug may be shed into the blood and plasma.2 |
Customer Reviews

FAQs
How do you ensure your rAAV vectors are safe for use in preclinical and clinical studies?
We prioritize safety and quality at every stage. Our proprietary capsid design and screening processes are specifically aimed at identifying variants that not only have high potency but also exhibit favorable biodistribution to minimize off-target effects. Our vector production and purification processes are rigorous, ensuring high purity and low endotoxin levels, which are essential for in vivo safety.
What makes your AAV design service better than using a standard, off-the-shelf serotype?
Standard serotypes like AAV5 and AAV9 may not be optimal for targeting specific cell types or deep brain structures with high efficiency. Our service leverages unbiased screening to identify novel capsids with superior tropism and potency, as demonstrated in published data. This allows for lower dosing, which improves safety and reduces the overall cost of your therapeutic development.
How long does the entire process take, and what factors might influence the timeline?
While the typical timeframe is 8 to 16 weeks, the duration can vary. Factors that might influence the timeline include the complexity of the desired capsid, the specific therapeutic payload being delivered, and the extent of the in vivo validation required. We work closely with our clients to provide a realistic and transparent project plan from the outset.
What kind of data will I receive at the end of the project to validate the vector's performance?
We provide a comprehensive data package to support your research. This includes detailed reports on vector titer and purity, as well as in-depth analysis of in vitro transduction efficiency and specificity. For projects with in vivo studies, we also provide data on vector biodistribution, therapeutic gene expression, and efficacy in the specified animal model.
Contact Our Team for More Information and to Discuss Your Project
Reach out to discover how our expertise accelerates your HD and neurodegenerative disorder R&D. Our team stands ready to provide detailed information and a customized project proposal for your specific needs.
References
- Aqel, Sarah, et al. "Advances in Huntington's disease biomarkers: a 10-year bibliometric analysis and a comprehensive review." Biology 14.2 (2025): 129. https://doi.org/10.3390/biology14020129. Distributed under Open Access license CC BY 4.0, without modification.
- Spronck, Elisabeth A., et al. "Intrastriatal administration of AAV5-miHTT in non-human primates and rats is well tolerated and results in miHTT transgene expression in key areas of Huntington disease pathology." Brain Sciences 11.2 (2021): 129. https://doi.org/10.3390/brainsci11020129. Distributed under Open Access license CC BY 4.0, figures were cropped.
