GTOnco™ Cytokine Response Assay (In Vivo)
Generally, cytokine production, to a considerable extent, controls an immune response. Therefore, the characterization of cytokine release is important for understanding the nature of the immune response. However, it has been difficult to directly measure in vivo cytokine release because many cytokines are catabolized, utilized, or excreted shortly after they are produced, such as IL-4 and IFN-γ. To better determine in vivo cytokine production, Creative Biolabs has successfully established several cytokine response assay methods to detect the cytokine secretion in vivo. GTOnco™ cytokine response assays provide important and useful information and the results can reflect in vivo cytokine release by the whole animal rather than a single organ.
Cytokine Response Assay Introduction
Cytokine response assays are a crucial methodological framework in biomedical research and therapeutic development, providing indispensable insights into immune system function and its dysregulation. These assays enable researchers to quantitatively measure the production and activity of cytokines—small molecular signaling proteins that mediate and regulate immunity, inflammation, and hematopoiesis. In vivo cytokine response assays can specifically study these molecular events within the complex physiological environment of an organism, offering greater clinical relevance compared to in vitro systems.
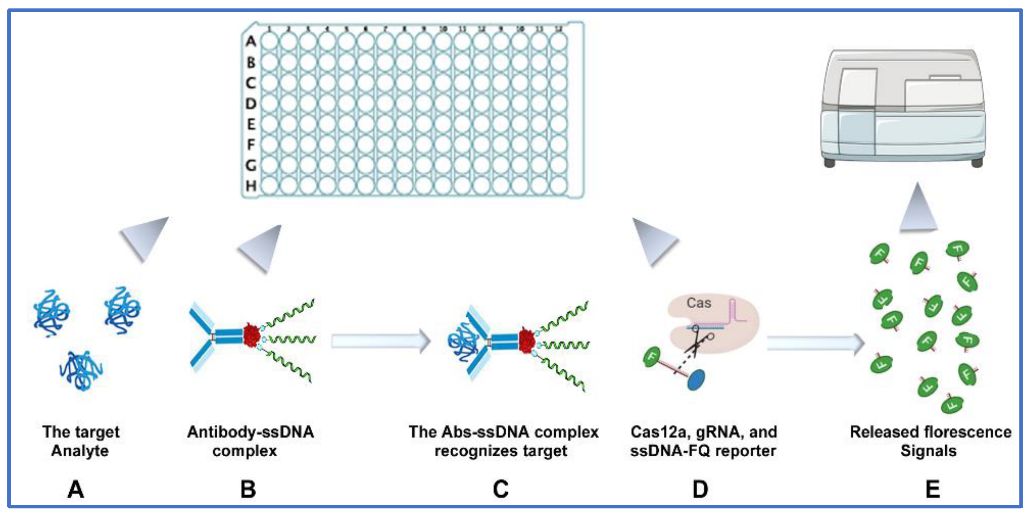 Figure 1 The workflow of CRISPR-ELISA assay to measure multiple cytokines.1
Figure 1 The workflow of CRISPR-ELISA assay to measure multiple cytokines.1
In vivo models consider the following factors:
- Pharmacokinetics (PK) and pharmacodynamics (PD): Drug distribution, metabolism, and concentration gradients at the site of action.
- Cellular microenvironment: The intricate interactions between different immune cell subsets (T cells, B cells, macrophages, dendritic cells) and non-immune cells in the native tissue microenvironment.
- Feedback loops: Complex, dynamic, and often nonlinear pro-inflammatory and anti-inflammatory signaling cascades that define the responses of living systems.
Cytokine Types and Their Functional Relevance
Cytokines comprise a diverse range of signaling molecules, which can be classified based on their structural characteristics, cellular origin, and functional properties. Understanding this classification system is crucial for interpreting cytokine response data and designing appropriate assays. The main functional categories include pro-inflammatory cytokines, anti-inflammatory cytokines, chemokines, and growth factors, each playing distinct roles in immune coordination and pathological processes.
| Cytokine Category | Representative Members | Primary Cellular Sources | Major Functions |
|---|---|---|---|
| Pro-inflammatory Cytokines | IL-6, TNF-α, IL-1β | Macrophages, T cells, Endothelial cells | Promote inflammation, Fever, Acute phase response |
| Anti-inflammatory Cytokines | IL-10, IL-4, IL-13 | Treg cells, M2 macrophages | Suppress inflammation, Promote tissue repair |
| Chemokines | IL-8, MCP-1, RANTES | Various immune and non-immune cells | Leukocyte recruitment and positioning |
| Growth Factors | G-CSF, GM-CSF, VEGF | Stromal cells, Endothelial cells | Hematopoiesis, Angiogenesis, Tissue remodeling |
Pro-inflammatory Cytokines Response Assay in Safety Studies
Controlled pro-inflammatory amplification is a fundamental immune mechanism, and its dysregulation can lead to a variety of pathological conditions.
Cytokine Release Syndrome (CRS) Model
CRS, often referred to as a "cytokine storm," is a potentially fatal systemic inflammatory response.
- Model Selection: Non-human primate (NHP) models or humanized mouse models are the preferred models for assessing human-specific cytokine-induced kinetics.
- Kinetic Assessment: Peak concentrations (Cmax) and time to peak concentrations (Tmax) of key inflammatory mediators are tracked via continuous blood sampling after dose escalation.
- Endpoint Relevance: Cytokine levels are closely correlated with toxic signs (e.g., fever, hemodynamic instability, lymphopenia) to determine actionable thresholds.
Methods of Cytokine Response Assay (In Vivo)
Accurate measurement of cytokine responses in living systems requires a combination of advanced detection platforms and appropriate experimental design. We have developed a multifaceted approach that leverages the unique strengths of different technologies to provide comprehensive cytokine profiling data.
Multiple Immunoassay Platforms
For broad-spectrum cytokine screening, we employ multiple immunoassay platforms capable of simultaneously quantifying up to 50 cytokines from very small sample sizes. These systems offer extremely high sensitivity (typically detecting concentrations in the pg/mL range) and maintain a wide dynamic range, capturing physiologically relevant cytokine concentrations.
ELISA-Based Quantitative Analysis
When precise quantification of specific cytokines is required, we utilize validated ELISA systems whose performance characteristics meet research standards. Optimized sample dilutions reduce matrix interference, making these assays particularly reliable for complex biological samples, including serum, plasma, and tissue homogenates.
Functional Cell Assays
In addition to simple cytokine concentration measurements, we have implemented functional assessment systems, including intracellular cytokine staining, to evaluate cytokine production at the single-cell level. These methods can provide crucial information about the frequency of cytokine-producing cells in heterogeneous cell populations, which is not available through batch measurement methods.
A Flexible Suite of Services at Creative Biolabs
Currently, GTOnco™ offers a comprehensive range of in vivo cytokine detection methods, with the in vivo cytokine capture assay (IVCCA) as a core service. IVCCA enhances detection sensitivity by extending the half-life of secreted cytokines—especially short-half-life ones like IL-2, IFN-γ, and TNF-α—via injection of biotinylated neutralizing anti-cytokine monoclonal IgG antibodies. These antibodies bind target cytokines to inhibit utilization, degradation, and excretion, accumulating cytokine-mAb complexes in the blood for subsequent quantification. We integrate multiple validated techniques: ELISA for precise single-cytokine quantification, multiplex immunoassays for simultaneous profiling of up to 50 cytokines, and functional cell assays (e.g., intracellular cytokine staining) for single-cell level analysis. Our services support both absorbance and luminescence-based measurements, and can be customized to meet specific research needs.
Multi-species Assay
We are proficient in all standard preclinical models, including mice, rats, guinea pigs, rabbits, non-human primates (NHP), and humanized models, and have validated assay kits for each species.
Customized Matrix Processing
We routinely analyze complex biological matrices, including bronchoalveolar lavage fluid (BALF), synovial fluid, cerebrospinal fluid (CSF), tissue homogenates, and high-viscosity tumor lysates.
Kinetic Study Design
Our experienced team of PhD scientists collaborates to develop optimal study protocols, including appropriate dosing regimens, consecutive sampling time points, and statistical power analysis.
Features of Our Cytokine Response Assay at GTOnco™ Platform
- Measurement of specific cytokines, including IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, etc.
- The detection of total-body cytokine secretion is available and efficient.
- Our assay does not require the sacrifice of the animal to obtain tissue samples.
Our Collaboration Process for Client Success
At Creative Biolabs, we view each client as a strategic partner with shared goals and consistent success metrics. Our collaborative process is designed to ensure that cytokine response assessment is seamlessly integrated into your overall R&D strategy while maintaining flexibility to meet evolving research needs.
-
Phase 1
Needs Assessment and Protocol Design
We begin each collaboration with a comprehensive exploration process, focusing on understanding your specific research objectives, regulatory requirements, and practical limitations. Our research team then develops a customized testing strategy to align cytokine response endpoints with your overall R&D goals, including selecting appropriate model systems, relevant time points, and best analytical methods.
-
Phase 2
Model Development and Validation
We build and validate disease-specific models (with appropriate challenges) based on your research context. This model validation includes verifying cytokine production dynamics and demonstrating pharmacological responses to reference compounds, ensuring the robustness and reproducibility of the system used for therapeutic assessment.
-
Phase 3
Assay Implementation and Data Generation
Our technical team performs cytokine response assessments using validated methods and rigorous quality control procedures. During this phase, we maintain transparent communication, regularly updating research progress and preliminary observations to inform subsequent experiments.
-
Phase 4
Data Interpretation and Reporting
We provide more than just raw data; our team offers expert interpretation, analyzing cytokine response results within the context of disease biology and treatment mechanisms. The final report includes comprehensive datasets, statistical analyses, and strategic insights.
Frequently Asked Questions
Q: What are the most common sources of variation detected in in vivo studies?
A: The main sources of variation are biological factors (inter-animal variability) and preanalytical factors. We reduce preanalytical variation by strictly controlling sample collection (e.g., immediate centrifugation, use of specific anticoagulants/protease inhibitors, -80°C freezing protocols), which is crucial for the stability of unstable cytokine proteins.
Q: How do you address the challenges of cytokine redundancy and pleiotropic effects in data interpretation?
A: We employ a network-based analytical approach to assess cytokine co-regulatory patterns, rather than focusing solely on individual mediators. This systemic perspective helps distinguish meaningful biological signals from accompanying phenomena. Furthermore, we frequently integrate cytokine response data with complementary endpoints, including cell phenotype analysis and functional assays, to establish mechanism-response relationships.
Q: Which sample types do you support for cytokine response assessment?
A: Our methods have been validated against a variety of sample matrices, including serum, plasma, tissue homogenates, bronchoalveolar lavage fluid, and cell culture supernatants. We optimize sample processing protocols for each matrix to minimize pre-analytical variability and maintain cytokine stability.
Q: Can you develop custom cytokine portfolios for specific therapeutic areas?
A: Yes, we regularly design cytokine portfolios for specific therapeutic areas, focusing on cytokines with a clear association with specific disease processes. These custom analytical panels typically contain 10–15 carefully selected analytes to comprehensively cover key pathological pathways while maintaining analytical performance.
Connect with Us Anytime!
Creative Biolabs possesses the qualification to supply GLP-compliant in vivo assay for the preclinical evaluation of gene therapy-based I-O agents. GTOnco™ platform can provide one-stop services for your studies of cytokine cross-talk, regulation of cytokine production, cytokine regulation of inflammatory conditions and cytokine-mediated host protection against infection. Please contact us to discuss your demands or to request a proposal.
Reference
- Li N, Chinthalapally M, Holden V K, et al. Profiling plasma cytokines by A CRISPR-ELISA assay for early detection of lung cancer. Journal of Clinical Medicine, 2022, 11(23): 6923. https://doi.org/10.3390/jcm11236923 (Distributed under Open Access license CC BY 4.0, without modification.)
