GTOnco™ T Cell Persistence & Trafficking Assay Service
T persistence and trafficking properties are important factors affecting the anti-tumor activity of I-O products. Generally, after adoptive T cell transfer, the T cell persistence is likely to promote long-term anti-tumor effects. In addition, following antigen-specific stimulation, the trafficking properties of T cells are changed and these activated and effector T cells acquire the ability to effectively and specifically home to other organs. Based on the outstanding expertise and rich experience, Creative Biolabs has developed the fast, reliable and affordable T cell persistence & trafficking assay platform to support your gene therapy-based I-O drugs development. We also provide one-stop preclinical in vivo service for our clients to evaluate the anti-tumor effects of I-O products, especially in the field of CAR-T or TCR-T therapy.
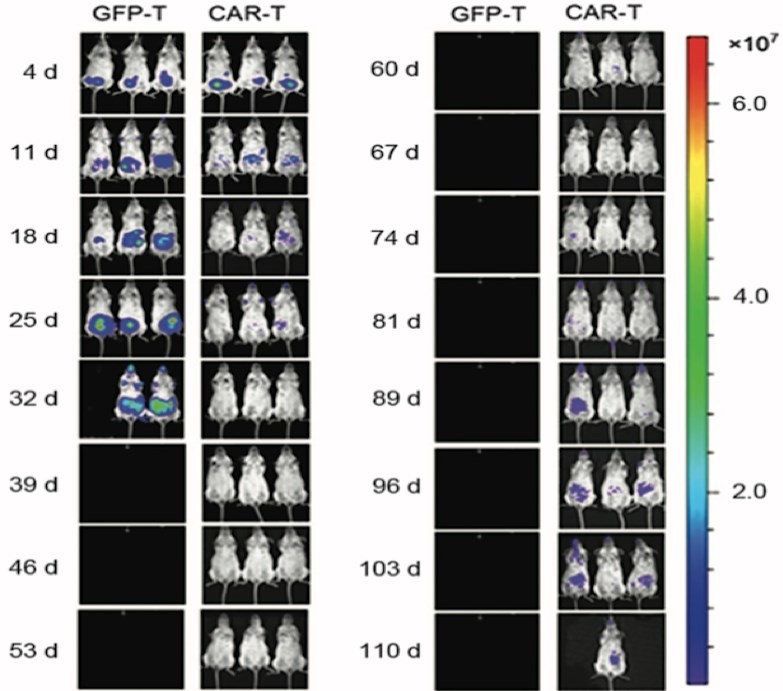 Figure 1. The T Cell Persistence and Trafficking Assay In Vivo.1
Figure 1. The T Cell Persistence and Trafficking Assay In Vivo.1
Background
Cancer immunotherapy, which utilizes the body's own immune system to fight malignant cells, has revolutionized oncology, with T-cell-mediated therapies at the forefront of this treatment paradigm. However, the clinical efficacy of these therapies is inconsistent, particularly in the treatment of solid tumors, where insufficient T-cell infiltration and limited functional durability remain significant obstacles. Recent data show that less than 10% of patients with solid tumors have a durable response to current T-cell therapies, highlighting the urgent need to develop advanced analytical tools capable of accurately assessing and optimizing T-cell behavior under physiologically relevant conditions.
T Cell Trafficking Flow Cytometry
Flow cytometry is an indispensable tool for characterizing T cell migration, as it goes beyond simple cell counting to delve into the molecular mechanisms regulating T cell migration. Multiparameter flow cytometry typically uses a detection plate with more than 10 colors to simultaneously detect these markers on CD4+ and CD8+ cell subsets in isolated tumor tissue. For example, high expression of CCR7/CD62L on tumor-infiltrating lymphocytes (TILs) is generally a negative marker, indicating that these cells retain a lymphocyte-homing phenotype rather than an active tumor microenvironment (TME)-homing phenotype. Conversely, in various solid tumor models, T cells expressing high proportions of TME chemokine receptors, such as CXCR3 and CCR5, are strongly associated with effective tumor invasion and a favorable clinical prognosis. This involves multicolor staining, primarily targeting adhesion molecules and chemokine receptors—collectively known as homing receptors.
Table 1: Key markers analyzed in GTOnco™ T cell trafficking flow cytometry
| Marker Category | Specific Markers | Biological Significance |
|---|---|---|
| Activation Markers | CD39, CD69, CD279 (PD-1) | Indicate recent T cell activation and potential exhaustion states |
| Effector Molecules | Granzyme B, Perforin, IFN-γ | Measure cytotoxic potential and functional capability |
| Trafficking Receptors | CCR7, CXCR3, CD62L | Determine homing potential to lymph nodes or inflamed tissues |
| Differentiation Markers | CD45RA, CD45RO, CD95 | Distinguish naive, effector, and memory populations |
Technology of T Cell Persistence & Trafficking Assay
GTOnco™ T-cell persistence and migration analysis employs a multimodal approach, integrating multiple cutting-edge technologies to comprehensively and deeply elucidate T-cell behavior. At the heart of this platform is a modular nanobody system derived from single-domain antibodies, which exhibits superior tissue penetration and molecular specificity compared to traditional antibodies.
Proximity Labeling Mapped to the Microenvironment
GTOnco™ analysis incorporates groundbreaking research from leading institutions, integrating a novel proximity-dependent antigen labeling system that amplifies T-cell recognition signals at the tumor interface. This innovative approach addresses the fundamental challenge of low antigen density, which typically limits the efficacy of T-cell therapy for solid tumors. By precisely constructing artificial antigen clusters on the tumor cell surface, this system significantly enhances T-cell activation and cytotoxicity without requiring genetic modification of target cells. This technology is particularly suitable for:
- Amplifying weak tumor antigens to levels sufficient for effective recognition by T cells
- Constructing synthetic neoantigens on the tumor surface for targeted binding
- Spatially restricting T cell activation to minimize off-target effects on healthy tissues
- Studying the dynamic changes in immune synapse formation and stability
High-Dimensional Immune Monitoring
The GTOnco™ platform integrates comprehensive immune receptor sequencing technologies to track the clonal dynamics and functional status of T cells throughout their lifecycle. Through collaboration with Adaptive Biotechnologies, our assay utilizes deep sequencing of T cell receptors (TCRs) to monitor:
- Receptor repertoire diversity indicators
- Shared common clones
A Flexible Suite of Services at Creative Biolabs
Creative Biolabs offers much more than just test kits; we provide a comprehensive end-to-end service designed to seamlessly integrate with your drug discovery and development process. Our team of PhD-level scientists and immunotherapy experts collaborates with you to design, execute, and analyze studies to generate actionable data, reduce project risk, and accelerate development. Our service portfolio addresses key challenges in the development of T-cell therapies for cancer.
Customized Experimental Design and Consultation
We begin by gaining a deep understanding of your specific scientific questions and project objectives. Our services include:
- Scope Definition Meetings: Through collaborative meetings, we clarify key objectives, such as comparing the persistence of different CAR constructs, optimizing culture conditions to enhance cell migration, or benchmarking your T-cell product against competitors.
- Customized Assay Configurations: We customize GTOnco™ platform modules to meet your needs, selecting appropriate patient-derived organoid models, flow cytometry assay combinations, and longitudinal imaging endpoints.
T Cell Persistence Analysis Service
- Longitudinal Co-culture Studies: Monitoring T cell survival, metabolic activity, and phenotypic drift over 14–28 days in the presence of tumor organoids.
- Exhaustion and Senescence Biomarker Tracking: Quantitative analysis of biomarkers such as PD-1, LAG-3, TIM-3, and CD39 to assess functional persistence.
- Metabolic Adaptability Assessment: Assessing oxidative phosphorylation and glycolytic capacity using Seahorse XF technology and correlating metabolic profiles with persistence.
T Cell Migration and Infiltration Analysis Service
- 3D Migration and Invasion Assays
- Chemotaxis and Adhesion Analysis
- In vivo transport validation (optional)
High-parameter phenotypic and functional characterization services:
- Advanced flow cytometry
- Multiple cytokine secretion analysis
Data Analysis, Bioinformatics, and Reporting
We transform complex raw data into clear and understandable insights:
- Integrated data analysis: Our bioinformatics team uses advanced algorithms for high-content image analysis, flow cytometry data clustering, and multi-omics dataset integration.
- Customized Report: You will receive a comprehensive final report including an executive summary, detailed methodology, raw data, and strategic interpretation, providing recommendations for your next steps.
- Data Delivery Portal: Secure and reliable cloud access for easy access to all project data, visualizations, and analysis documents.
Our Collaboration Process
- Initial Consultation: We discuss your project needs and sign a confidentiality agreement (CDA).
- Proposal and Agreement: We provide a detailed project proposal including timelines and cost estimates.
- Sample Transportation: You will ship your T-cell products and/or tumor samples to our state-of-the-art laboratory.
- Project Initiation and Experiment Execution: We will assign a dedicated project manager and initiate your customized study.
- Preliminary Data and Mid-Term Progress: We will provide regular updates on the experimental progress.
- Final Data Delivery and Consultation: We will deliver the final report and hold a project summary meeting to discuss the research results and their significance.
Customer Review

"Our lab extensively utilizes the GTOnco™ platform to investigate CAR-T therapy resistance mechanisms in pancreatic cancer models. The high-content imaging component revealed unexpected heterogeneity in T cell infiltration patterns within patient-derived organoids, a feature completely overlooked by conventional Transwell experiments. Most notably, we found that a small subset of T cells with moderate CXCR3 expression levels (rather than the highest-expressing T cells) exhibited stronger organoid core penetration. This counterintuitive finding, subsequently validated in in vivo experiments, fundamentally changes our approach to constructing T cells for solid tumor therapy."
— Dr. Elena Rodriguez, the lead researcher from a leading cancer center
Frequently Asked Questions
Q: How does the GTOnco™ assay differ from other T-cell function assays?
A: The GTOnco™ platform uniquely integrates patient-derived 3D organoids, nanobody-enhanced assays, and advanced computational analysis to resolve T-cell behavior under physiological conditions with unprecedented resolution. Unlike traditional assays that measure single parameters in isolation, GTOnco™ captures the dynamic interactions between T-cell persistence, migration, and function by longitudinally monitoring models that faithfully reproduce key features of human tumors. This system-level approach detects emergent properties and compensatory mechanisms that are undetectable by reductionist methods.
Q: What are the sample requirements for the GTOnco™ assay?
A: The platform is compatible with a variety of sample types, including primary human T cells (resting or activated), cryopreserved peripheral blood mononuclear cells (PBMCs), and preclinical samples from mouse models. For cell migration studies using patient-derived organoids, we recommend obtaining tumor tissue fragments or isolated cells for expansion in our standardized bioreactor system. Our technical team provides comprehensive support for sample optimization to ensure robust detection performance across diverse starting materials.
Q: How does the GTOnco™ platform address the heterogeneity of T-cell products?
A: The GTOnco™ platform leverages the single-cell resolution provided by our high-parameter flow cytometer and imaging components to precisely capture and quantify heterogeneity within T-cell populations. Advanced clustering algorithms identify functionally distinct subpopulations based on the co-expression of multiple markers, enabling researchers to understand how different subpopulations contribute to overall therapeutic efficacy. This capability is particularly important for optimizing manufacturing processes to enrich the desired T-cell phenotype.
Connect with Us Anytime!
Leveraging our industry expertise, Creative Biolabs provides the advanced in vivo assay platform for our clients' projects research. We are committed to offering the technologies and resources to facilitate your gene therapy-based I-O drugs development and boost the progress to clinical trials. Contact us today for a proposal to support all of your immuno-oncology research needs.
Reference
- Bai, Y.; et al. (2015). Erratum: Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discovery. 2(1). 10.1038/celldisc.2015.40 (Distributed under Open Access license CC BY 4.0, without modification.)
