GTOnco™ Immune Cell-Mediated Tumor Regression Assay Service
Preclinical therapeutic efficacy test of gene therapy-based I-O products can provide useful and valuable data for their later clinical application. The anti-tumor activity of I-O drugs mediated by immune cells is the core content of efficacy test. The cytotoxic T lymphocyte (CTL) has been proposed as the primary effector function necessary for tumor regression, especially for the CD8+ T cell cytotoxic activity. With years of preclinical research and project management experience, Creative Biolabs has developed various robust animal models and related assays to greatly facilitate I-O therapy development. We are capable of conducting many tests to evaluate the immune cell-mediated tumor regression efficacy in I-O drugs therapy.
Introduction of Immune Cell-Mediated Tumor Regression Assay
Immune cell-mediated tumor regression assay (ICM-TRA) is a leading functional assay in the field of immuno-oncology. It goes beyond simple binding affinity, directly measuring the ultimate therapeutic target: the killing effect of immune effector cells on tumor cells. The design of this assay requires strict control over cell source, the ratio of effector cells to target cells, and the selection of detection indicators to accurately simulate the complex environment.
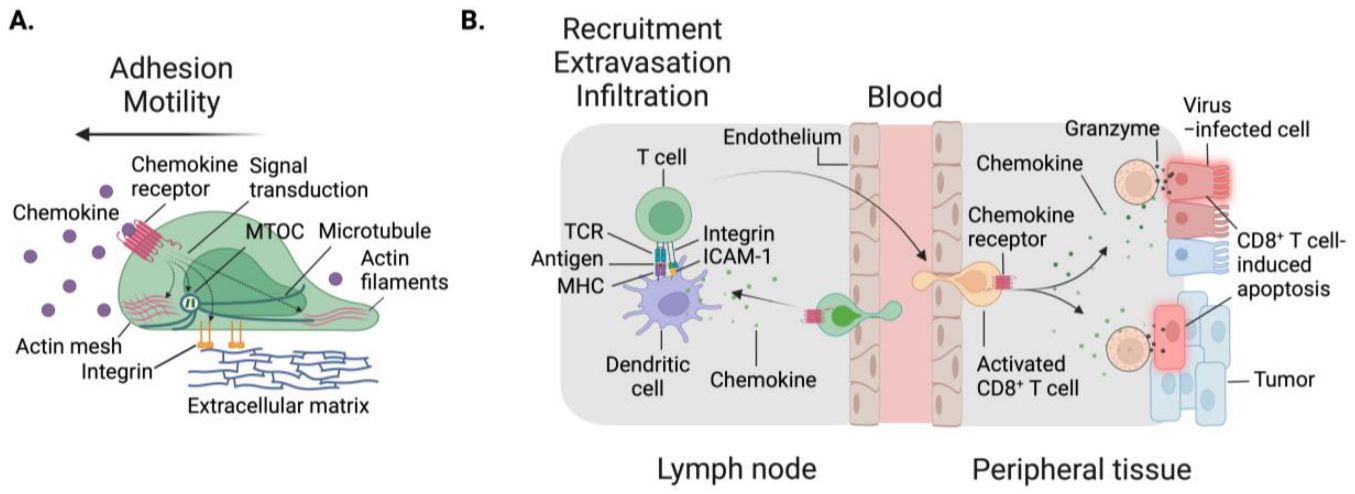 Figure 1. Cellular migration is dependent on cell intrinsic factors, as well as multiple signal inputs from the environment.1
Figure 1. Cellular migration is dependent on cell intrinsic factors, as well as multiple signal inputs from the environment.1
Why Need Immune Cell-Mediated Tumor Regression Assay?
The emergence of immuno-oncology (IO) has profoundly transformed the paradigm of cancer treatment, leveraging the power of a patient's own immune system to identify and eliminate malignant cells. At the heart of this therapeutic revolution lies immune cell-mediated tumor regression, a complex process involving the synergistic action of multiple immune effector cells. The ability to accurately and reliably quantify these cell interactions is crucial for the preclinical and clinical validation of novel immunotherapies, including immune checkpoint inhibitors, chimeric antigen receptor (CAR) T cells, tumor vaccines, and bispecific antibodies.
Key parameters in a comprehensive tumor regression assay would include:
Cellular lysis activity
The main functional readout, often measured by release of one or more cellular markers (e.g., lactate dehydrogenase (LDH) or proteolytic cleavage of fluorescently labeled caspase). Alternatively, direct cell counting can be used to assess live and dead cells using flow cytometry.
Immune cell activation and phenotype
The expression of activation markers (e.g. CD69, CD25), checkpoint receptors (e.g. PD-1, LAG-3) and/or intracellular cytokines (e.g. IFN-γ, TNF-α) can be measured in the effector cells after co-culture.
Immune synapse quality
A high-content image-based readout can be used to examine the quality of the immune synapse formed between the effector and the target cell, which is an important contributor to killing efficiency.
Cytokine secretion profile
Multiplex ELISA of the culture supernatant provides a broader view of the immune response, allowing for the distinction between a pro-inflammatory environment (e.g. IL-2, IFN-γ, IL-12) and a suppressive one (e.g. IL-10, TGF-β).
Applications of Immune Cell-Mediated Tumor Regression Assays
These assays are an integral part of multiple stages of therapeutic drug development:
- Biopharmaceutical Screening and Efficacy Assessment: Quantifying the antibody-dependent cell-mediated cytotoxicity (ADCC) or antibody-dependent phagocytic activity (ADCP) of therapeutic antibodies (e.g., trastuzumab, rituximab).
- CAR-T/TCR-T Cell Therapy Development: Evaluating the killing efficacy, kinetics, and specificity of engineered T cells against various target tumor cell lines before conducting trials.
- Immune Checkpoint Inhibitor (ICI) Assays: Assessing the ability of ICIs (e.g., anti-PD-1, anti-CTLA-4) to restore or enhance endogenous T cells, and the activity of NK cells against autologous or allogeneic tumor cells.
- Vaccine Development: Measuring antigen-specific T cell cytotoxicity induced by novel cancer vaccine candidates.
A Flexible Suite of Services at Creative Biolabs
The techniques at GTOnco™ platform can provide a high-quality therapeutic efficacy test service for our customers. Our flow cytometry-based assay can be designed to quantitatively measure the immune cells from peripheral blood or harvested tissues, such as bone marrow, liver and spleen by detecting their specific targets and markers. The advanced imaging system is able to perform the non-invasive study and monitor the tumor progression in animal models. We also provide histologic and pathologic service to validate the presence and infiltration of tumor cells and perform the morphometric and visualization analysis. Of course, the animal and tumor observation including behavior, body weight, tumor size and morphology will be collected for the evaluation of immune cell-mediated tumor regression.
Comprehensive Cytotoxicity Assays
We employ a multi-platform approach to assess immune-mediated tumor killing, including lactate dehydrogenase (LDH) release assays, real-time live-cell imaging, flow cytometry-based cytotoxicity measurements, and high-content image analysis.
Customized Immune Cell Engineering
Our experts focus on constructing genetically modified immune cells with enhanced tumor-killing capabilities, including CAR-T cells, TCR-T cells, NK cells, and macrophages.
Immune Checkpoint Inhibitor Evaluation
We utilize specialized assays to evaluate the efficacy of immune checkpoint inhibitors, including monotherapy and combination therapy.
Tumor Microenvironment Analysis
We comprehensively characterize the tumor immune microenvironment post-treatment using techniques such as multiplex immunofluorescence, cytokine profiling, immune cell phenotyping, and spatial transcriptomics.
Pharmacodynamic Biomarker Development
We identify and validate target-binding and bioactivity-related biomarkers to support translational trial design. Our biomarker services help translate preclinical research findings into applications.
Immune Cell-Mediated Tumor Regression Assay Methods
Accurately quantifying the cytotoxicity of immune cells is crucial. Various methods have been employed, differing in throughput, sensitivity, and mechanistic details.
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Chromium-51 (Cr51) Release Assay | Measures the release of radioactive Cr51 from lysed target cells. | Gold standard, highly sensitive, reliable. | Use of radioisotopes, limited mechanistic insight, short half-life. |
| Lactate Dehydrogenase (LDH) Release Assay | Measures LDH, a stable cytosolic enzyme, released upon cell lysis. | Non-radioactive, high throughput, simple. | Sensitive to non-specific cell damage, high background possible. |
| Flow Cytometry-Based Assays (e.g., CFSE/PI) | Uses fluorescent dyes (e.g., CFSE for proliferation, PI for dead cells) to identify target cells and measure viability/apoptosis. | Single-cell resolution, provides phenotypic data on immune and target cells, multiplexing capability. | Requires advanced equipment and expertise in data analysis. |
| Real-Time Imaging Cytotoxicity Assays | Uses time-lapse microscopy and fluorescent probes (e.g., for caspase activation or membrane integrity) to track killing kinetics. | Provides kinetic data (rate of killing), allows visualization of cell-cell interactions and mechanism (e.g., caspase activation). | Lower throughput, potentially more complex data handling. |
Features of Our Service at GTOnco™ Platform
- Outstanding expertise and rich experience to get rich and robust results
- Using diversified advanced analysis systems to meet different demands
- Fast turnaround time and feedback
Frequently Asked Questions
Q: Can your assays differentiate between various modes of cell death?
A: Yes. We can perform mechanistic analysis on immune effector-induced tumor cell death patterns by staining with specific fluorescent probes (e.g. Annexin V, PI, Caspase) using flow cytometry and imaging assays.
Q: What about samples with low cell number?
A: Yes. Our custom microfluidic and low-input platform can accommodate even precious patient-derived samples with as few as 20,000–40,000 cells for analysis. To extract the most information out of precious samples with limited cell numbers, we optimize the experimental design and condition for every experiment. For this reason, our services are especially applicable for those with rare primary specimens in particular.
Q: What strategies do you take to circumvent the problems caused by poor solubility and poor clearance of soluble antigens in targeted immunotherapy?
A: We use several engineering strategies, such as pH-dependent antibodies. In this system, the antibody dissociates from its antigen preferentially in endosomes with acidic pH to increase antigen clearance while it maintains a high binding affinity at neutral pH to increase target binding.
Q: What biomarkers do you recommend to measure the whole picture in immune surveillance?
A: We prefer to take a multi-level analysis approach, where we combine the use of functional cytotoxicity indicator with immunophenotypic analysis, cytokine profiling, and spatial analysis to generate a comprehensive assessment that more fully recapitulates the intensity and mechanism of the antitumor immunity and therefore a better picture of the therapeutic activity.
Connect with Us Anytime!
All our experiments are performed by well-trained and experienced technicians in a GLP-compliant and IACUC-regulated facility. By utilizing appropriate animal models, Creative Biolabs provides high-quality therapeutic efficacy test services to help our customers and expedite their IND application. If you have any related needs, please feel free to contact us. We look forward to working with you in the future.
Reference
- Ryan A T, Kim M, Lim K. Immune cell migration to cancer. Cells, 2024, 13(10): 844. https://doi.org/10.3390/cells13100844 (Distributed under Open Access license CC BY 4.0, without modification.)
