GTOnco™ DC Migration Assay Service
Dendritic cells (DCs) are central players of the immune system and the migration of DCs into secondary lymphoid organs and tissues plays a crucial role in the initiation of innate and adaptive immunity. Immature DCs with strong migration ability are activated by the hapten-protein complex and differentiated from antigen-capture and processing cells into potent immunostimulatory DCs to present antigen effectively to effector T-cells. Most DC driven responses require their migration to a certain target destination to promote immunity. Based on the outstanding expertise and rich experience, Creative Biolabs has the capability to provide high-quality DCs migration assay service to help our clients understand the pathogenesis of a range of diseases and accelerate their development of gene therapy-based I-O products and immuno-oncology research.
DC Migration Assay Introduction
The dynamic migration process of DCs is crucial for the initiation and regulation of immune responses. Acting as professional antigen-presenting cells (APCs), DCs act as sentinels, migrating from peripheral tissues (to capture antigens) to draining lymph nodes (DLNs) (to prime T cells). This migratory capacity is carefully regulated by chemokines and their cognate receptors, most notably the expression of CCR7 and its ligands CCL19 and CCL21 following DC maturation. The ability to accurately and robustly quantify this cell migration capacity, particularly through DC migration assays, is a key in vitro tool for advancing immunotherapy, vaccine development, and understanding immunopathology.
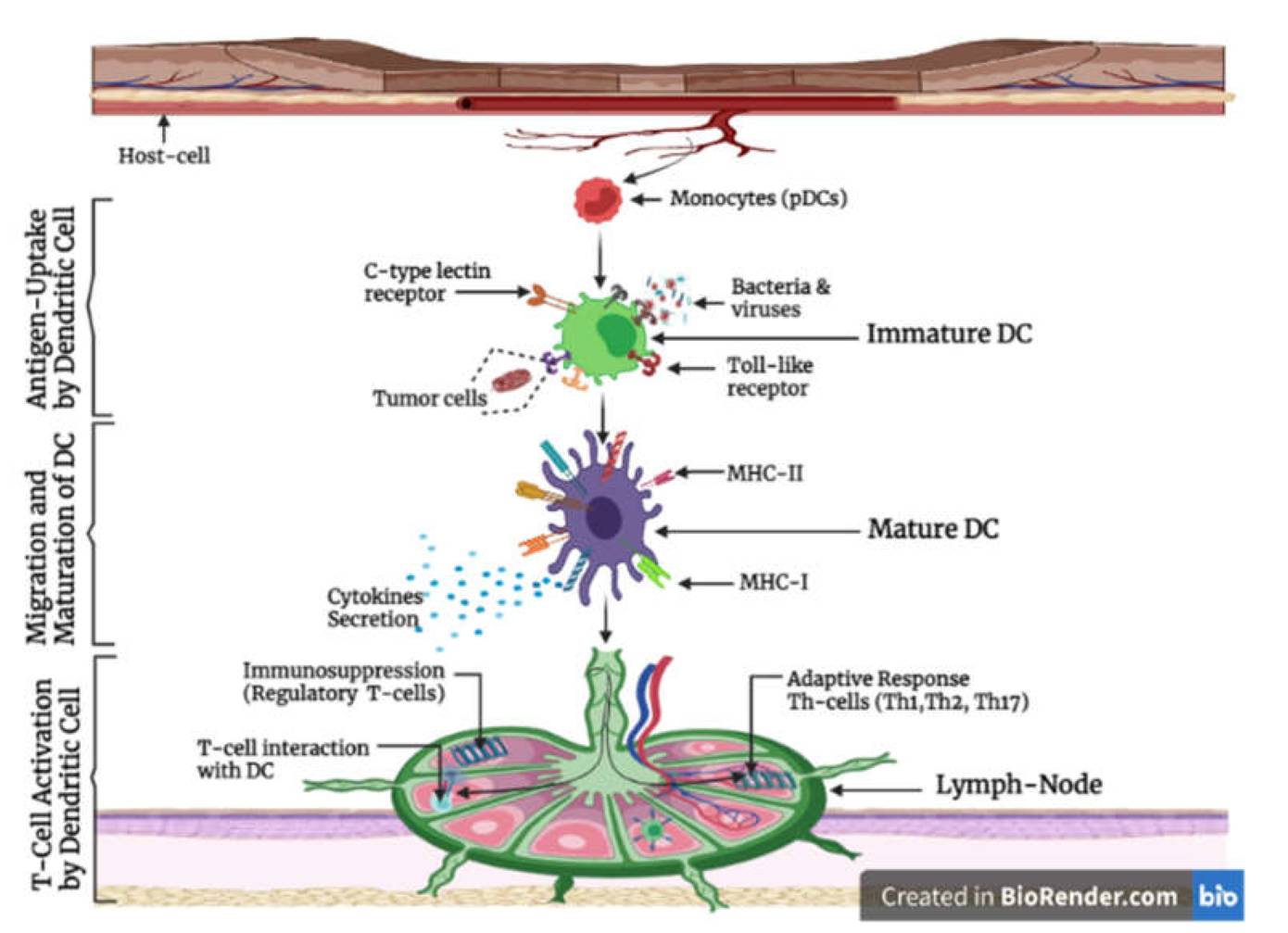 Figure 1 Antigen uptake, migration, maturation and T-cell activation by the dendritic cell.1
Figure 1 Antigen uptake, migration, maturation and T-cell activation by the dendritic cell.1
Transwell Migration Assay
The Transwell migration assay, also known as the Boyden chamber assay, has become a fundamental tool for studying dendritic cell migratory behavior in vitro. This system utilizes a two-chamber design separated by a microporous membrane, which acts as a physical barrier while allowing cells to migrate in response to a chemotactic gradient. The experimental setup involves seeding dendritic cells in the upper chamber, while the lower chamber contains chemoattractants, such as specific chemokines (e.g., CCL19, CCL21), formyl peptides, or serum components, which form a concentration gradient across the membrane. The choice of membrane pore size (typically 3-12 μm) is crucial and must be optimized based on the size and properties of the dendritic cells. Smaller pore sizes (3-5 μm) are suitable for studying chemotactic motility, while larger pore sizes (8-12 μm) are suitable for assessing complete migratory capacity.
Table 1: Comparison of Transwell Membrane Pores for Dendritic Cell Migration Studies
| Pore Size (μm) | Primary Applications | DC Subtypes Suitable | Key Considerations |
|---|---|---|---|
| 3.0 | Chemokinesis studies, small cell subsets | Plasmacytoid DCs, DC precursors | Restricted movement, minimal requirement for protrusion extension |
| 5.0 | General migration without protrusion requirements | Most conventional DCs | Balanced restriction and permeability |
| 8.0 | Standard migration and invasion assays | Monocyte-derived DCs, mature DCs | Permits cellular protrusions, suitable for most applications |
| 12.0 | Slow-moving or large DCs, transmigration studies | Primary astrocytes, large activated DCs | Ideal for cells with extensive processes or slow motility |
Mouse Dendritic Cell Migration Assay
While in vitro Transwell assays are powerful, translational validation of their results requires validation in relevant animal models. Mouse dendritic cell migration assays are a crucial complement. Antigen transport to lymph nodes: The primary in vivo endpoint is quantification of the number of antigen-loaded dendritic cells (DCs) that migrate from the injection site in peripheral tissues (e.g., skin, footpad) to draining lymph nodes (e.g., popliteal or inguinal lymph nodes).
Methods:
- Typically, ex vivo fluorescently labeled DCs (e.g., with CFSE or the lipophilic tracer Dil) are injected into mouse peripheral tissues.
- Alternatively, compounds/antigens are injected and the migration of endogenously activated DCs is tracked.
- After a defined period of time (e.g., 24-48 hours), DLNs are harvested, prepared into single-cell suspensions, and the number of migrated labeled DCs is quantified using flow cytometry.
Detection Methods for Dendritic Cell Migration Assays
| Detection Method | Principles | Advantages | Ideal Applications |
|---|---|---|---|
| Colorimetric (Crystal Violet) | Chemical staining of migrated cells | Cost-effective, simple instrumentation, compatible with standard microscopy | Endpoint analyses, large screening campaigns with limited budget |
| Fluorometric (Calcein-AM, DAPI) | Fluorescent labeling of cellular components | Enhanced sensitivity, easier automation, better signal-to-noise ratio | Low cell numbers, simultaneous multiplexing with phenotypic markers |
| Flow Cytometry | Quantitative analysis of migrated cells in suspension | Multiparameter phenotyping, highest statistical power, subset resolution | In vivo migration tracking, complex populations requiring immunophenotyping |
| Live-Cell Imaging | Temporal monitoring of migratory behavior | Kinetic data, migration path analysis, dynamic response assessment | 3D migration, mechanistic studies, microfluidic platforms |
Core Services at Creative Biolabs
Migration is truly at the heart of DCs biology and most of the migration events are controlled by specific molecular guidance cues, including chemokine-chemokine receptor system (e.g. CCR7, CCL19 and CCL21) and some factors interacting with the DCs cytoskeleton. Taking advantage of the GTOnco™ platform, Creative Biolabs provides customized, standardized, reliable and high-quality DCs migration assay services for clients across the world.
Our one-stop services cover all aspects of DC migration study, including maturation and mobilization of tissue-resident DCs, intranodal migration and positioning of DCs and the migration in the skin, intestines, lung, central nervous system (CNS), atherosclerosis and inflammatory diseases, etc. In addition, the research of DCs subset or surface markers and the routes, cues and molecular basis of DCs migration are also available at GTOnco™ platform. We will design assay protocols for customers and select the most appropriate method to advance your gene therapy-based I-O drugs development.
- Standard Transwell Chemotaxis Assay
- Compound Screening Assay
- DC Maturation and Migration Profile Analysis
- Subtype-Specific Migration
- Organ-Specific Migration Models
Reasons to Select Our Services
Choosing Creative Biolabs means partnering with a leader committed to accelerating your biomedical discoveries.
| Feature | Strategic Advantage for the Client |
|---|---|
| Comprehensive DC Sourcing | Access to human and mouse DCs (moDCs, primary DCs, established lines) ensures the best model for your specific research question (e.g., species-specific drug testing). |
| Custom Assay Development | Beyond the standard Transwell, we offer chemotaxis under shear stress models and real-time live-cell imaging assays to capture the kinetics and mechanics of migration, providing deeper mechanistic insight. |
| Integrated Immunological Services | Migration assays are seamlessly integrated with downstream services, such as T cell proliferation assays (Mixed Lymphocyte Reaction - MLR), to provide a complete picture of DC function—migration followed by T cell priming. |
| Translational Data Generation | Our data is structured for direct utility in regulatory submissions, grant applications, and publication, accelerating the path from bench to bedside. |
Quality Isn't an Option—It's Our Guarantee
In the scientifically rigorous field of dendritic cell migration research, quality assurance goes beyond simple procedural compliance—it is the fundamental foundation for building reliable, interpretable, and publishable data. Our unwavering commitment to methodological excellence permeates every aspect of our services, starting with critical preanalytical considerations. Our quality framework begins with a rigorous dendritic cell validation protocol, ensuring comprehensive immunophenotypic characterization (CD11c, MHC-II, CD80, CD86, CD40) and functional validation using mixed lymphocyte reaction assays before using dendritic cells in migration studies. This foundational step ensures that the cell substrates used in your experiments possess the appropriate maturation state and functional capacity to generate physiologically relevant migration data.
Frequently Asked Questions
Q: What is the significance of pore size for dendritic cells (DCs) in Transwell assays?
A: The standard pore size of 3.0 μm is crucial. It is small enough to prevent passive cell passage and instead requires active, directed movement of dendritic cells (amoeba-like motility). Larger pore sizes can cause non-motile cells to fall out, thus interfering with the measurement of true chemotaxis.
Q: How can we ensure the biological relevance of the chemokine gradient?
A: We perform preliminary titration experiments to determine the optimal, non-saturating concentration of chemokine in the lower chamber. This ensures the formation of a physiological and sustained concentration gradient, which is essential for chemotactic migration. The positive control consistently migrates toward the known optimal chemokine concentration, serving as a benchmark for biologically valid assays.
Q: How does the Transwell migration assay differ from the invasion assay?
A: Standard migration assays use bare membranes to assess cell migration toward a soluble chemokine, mimicking a basic chemotactic response. In contrast, invasion assays incorporate a barrier composed of extracellular matrix components (Matrigel, collagen) on the membrane surface, requiring DCs to exhibit proteolytic activity and deformability to penetrate this physiological barrier. Therefore, invasion assays simulate the more complex process of tissue penetration, including degradation of basement membrane components—a critical ability for DCs to enter and exit tissues during immune responses. We recommend performing invasion assays when studying DC behavior in contexts involving tissue remodeling, inflammation, or the tumor microenvironment.
Q: What controls should be included in a DC migration assay?
A: A well-established DC migration assay should include multiple control conditions to ensure accurate interpretation of the results. Essential controls include: (1) background migration control (no chemokine in the lower chamber) to assess random migration; (2) positive control (normalized chemokines, such as CCL19/CCL21 or serum for CCR7-mediated migration) to verify cell responsiveness; (3) inhibition control (using migration inhibitors, such as cytochalasin D) to confirm assay specificity; and (4) viability assessment to exclude toxic confounding factors. For invasion assays, an additional migration control (no matrix) should be included to distinguish between effects on invasion and changes in general motility. Our service protocol systematically integrates all necessary controls and performs appropriate replicates.
Don't Hesitate, Contact Us!
Our comprehensive service portfolio provides researchers with access to this full spectrum of migratory assessment platforms, coupled with deep expertise in experimental design and data interpretation across diverse biological contexts. Whether your objectives involve basic mechanism discovery, therapeutic target validation, or compound screening, our specialized approaches deliver the robust, physiologically relevant data required to advance your research programs. We are pleased to share our precious experience in immunotherapy with our clients. Please contact us to learn more information. Our team will get back to you as soon as possible.
Reference
- Zanna M Y, Yasmin A R, Omar A R, et al. Review of dendritic cells, their role in clinical immunology, and distribution in various animal species. International journal of molecular sciences, 2021, 22(15): 8044. https://doi.org/10.3390/ijms22158044 (Distributed under Open Access license CC BY 4.0, without modification.)
