Lentiviral Vector Development for Hematopoietic Stem Cell-based Gene Therapy
Based on the outstanding expertise and rich experience, Creative Biolabs offers our clients lentiviral vector design service in the most high-quality and cost-effective way. Particularly, hematopoietic stem cells (HSCs)-based gene therapy development is our featured service which is reliable and economical.
Hematopoietic Stem Cell-based Gene Therapy
HSCs are multipotent, self-renewing progenitor cells that develop from mesodermal hemangioblast cells. HSCs can be found in adult bone marrow, peripheral blood, and umbilical cord blood. Currently, the hematopoietic system has been treated as an ideal target for gene therapy because of the ease with which HSCs can be accessed for ex vivo gene manipulation, effective gene modification, and re-administration as an intravenous infusion. The engrafted HSCs ensure a steady supply of genetically engineered progeny potentially for the recipient's lifetime. Mature cells of different lineages may then reverse pathological conditions such as inherited immune deficiencies, blood and storage disorders, infections, and cancer.
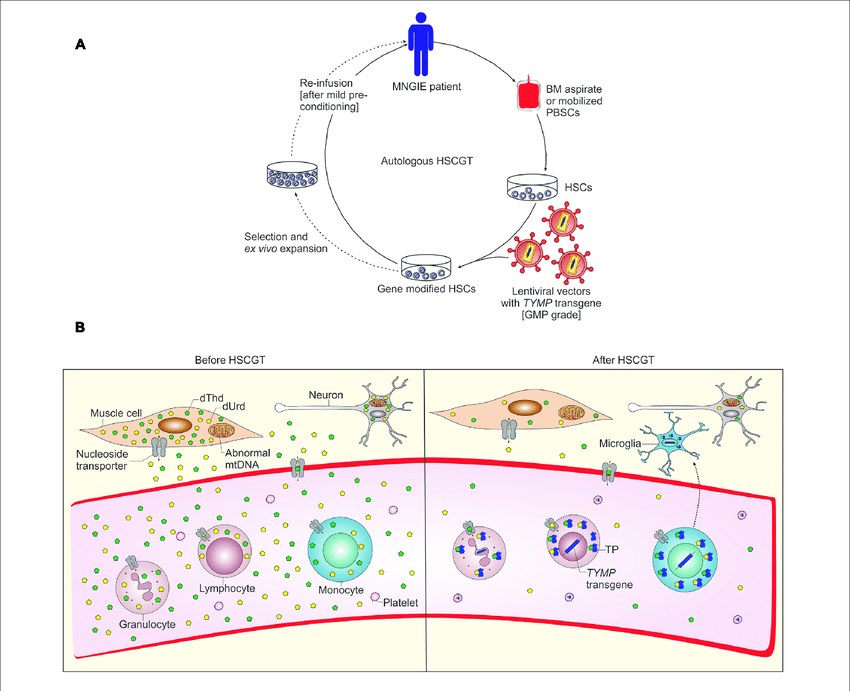 Figure 1. Schematic representation of autologous hematopoietic stem cell-based gene therapy for MNGIE and possible mechanism of biochemical correction by gene-modified HSCs. (Yadak, 2017)
Figure 1. Schematic representation of autologous hematopoietic stem cell-based gene therapy for MNGIE and possible mechanism of biochemical correction by gene-modified HSCs. (Yadak, 2017)
Application of HSCs-based Gene Therapy
Successful HSC gene therapy requires inserting the therapeutic gene into the cellular chromosomal DNA, or editing a selected nucleotide sequence, to ensure the maintenance of gene correction as the cell replicates its genome and transmits it to the progeny, whether during self-renewal or in the output of differentiating cell lineages. Recently, HSC gene therapy is becoming a powerful and versatile strategy to treat a growing number of human diseases. It has been reported that HSC gene therapy is very effective for the malignant hematologic disease but genetic diseases of the blood including immunodeficiencies, disorders of red cell production, or red cell function can also be corrected. Furthermore, HSC gene therapy can also correct some genetic diseases where a secreted enzyme, normally expressed in hematopoietic lineages can be taken up by surrounding affected cells.
Advantages
In the past, the most commonly used gene transfer tools rely on the ability of retroviruses to integrate into the chromatin of infected cells and are obtained by engineering the virus into replication-defective vehicles (vectors) of self-contained gene expression cassettes. Recently, a switch has been made to using lentiviral vectors (LV) based on HIV-1. It has been shown that LVs, compared to retroviral vectors, not only present higher efficiency for gene transfer, but also show a significantly decreased risk of genotoxicity, thanks to advanced vector design and the HIV integration pattern in the cell genome, which alleviates the risk of occurrence of oncogene activating insertions.
Working in the field of gene therapy for many years, Creative Biolabs is committed to offering high-quality lentiviral vector design service to promote the development of HSC-based gene therapy. Our professional scientists are happy to help you construct the most appropriate lentiviral vector targeting numerous diseases. For more detailed information, please feel free to contact us or directly send us a quote.
Reference
- Yadak, R.; et al. (2017). Mitochondrial neurogastrointestinal encephalomyopathy caused by thymidine phosphorylase enzyme deficiency: from pathogenesis to emerging therapeutic options. Frontiers in cellular neuroscience. p.31. Distributed under Open Access license CC BY 4.0, without modification.
