Lentiviral Vector Development for Sickle Cell Disease
Creative Biolabs' lentiviral vector development services encompass vector design, vector construction, process development, production, optimization and quality control testing. With extensive experience in lentiviral production and a strong focus on viral safety and consistency, Creative Biolabs ensures fast turnaround times and flexible batch production.
What Is Sickle Cell Disease?
SCD, also known as sickle cell anemia, is a group of genetic disorders that affect hemoglobin, the main protein in red blood cells that carries oxygen. SCD is caused by a mutation in the sixth amino acid of the hemoglobin (Hb) β-chain (E6V), affecting about 312,000 newborns worldwide annually. The mutation induces polymerization of Hb tetramers, red blood cell deformation, ischemia, anemia, and multiple organ damage. As a consequence, red blood cells (RBCs) lose flexibility and adopt the characteristic sickle shape in the capillary circulation.
Lentiviral Vector Introduction
Lentiviral vector (LV) is used in gene therapy and research applications. Vectors are engineered from lentiviruses like HIV, to provide the necessary genes into the host cells' genome. LVs have replication defects and can be designed for stable genetic integration into both dividing and non-dividing cells, which allows them to give long term transgenic expression.
Lentiviral Vector for Sickle Cell Disease Treatment
Currently, with the advent of HBB expressing LVs, LV-based gene therapy holds the promise of avoiding the need for suitable donors and reducing the morbidity and mortality associated with allogeneic transplantation. LV-based gene therapy strategies require the stable transfer of an anti-sickling HBB globin transgene and sustained expression of the therapeutic globin chain. Several LVs have been developed and tested in murine models of SCD and patients' hematopoietic stem progenitor cells (HSPCs). Moreover, pre-clinical and clinical studies have shown the potential of LV-based gene therapy in correcting the SCD phenotype. The LVs used in these studies express either the fetal γ-globin or mutant β-globins that interfere with axial and lateral contacts in the HbS polymer, thereby reducing HbS polymerization and sickling.
Current Treatments vs. Lentiviral Vector-Based Gene Therapy
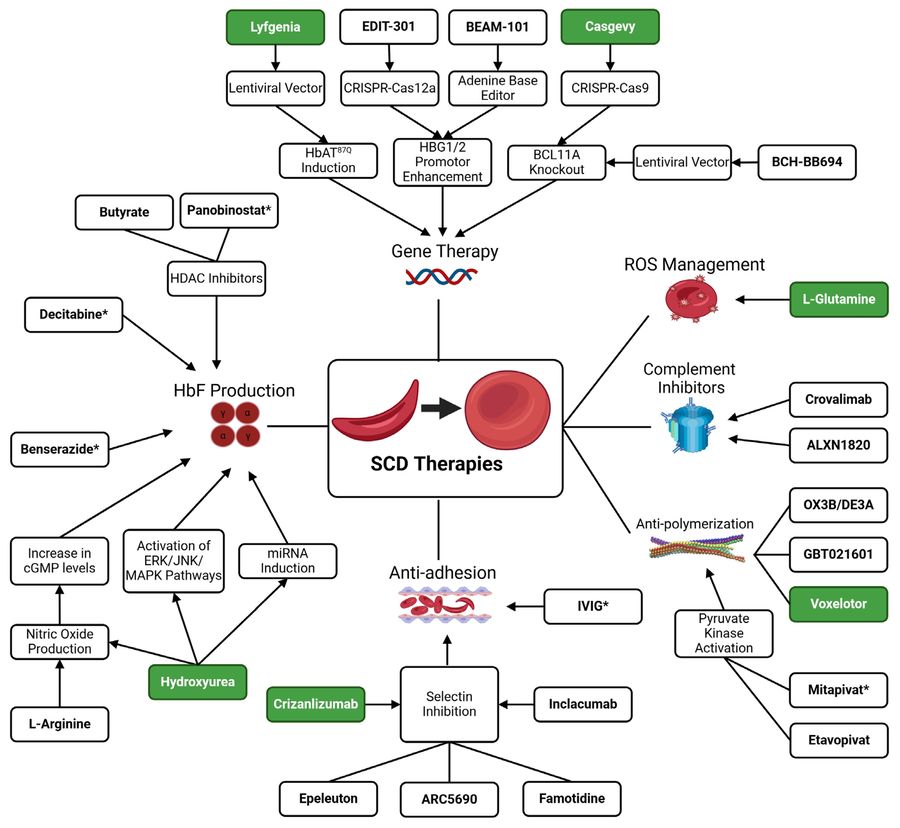 Figure 1 Approved and novel therapies and the associated pathways they affect in the treatment of SCD and its complications.1
Figure 1 Approved and novel therapies and the associated pathways they affect in the treatment of SCD and its complications.1
| Feature | Current Standard Treatments (e.g., Hydroxyurea, Transfusions) | Lentiviral Vector-Based Gene Therapy (e.g., LYFGENIA) |
|---|---|---|
| Mechanism | Symptom management: Increases HbF (hydroxyurea), provides healthy RBCs (transfusions). | Genetic correction: Introduces functional anti-sickling β-globin gene into autologous HSCs. |
| Treatment Frequency | Chronic, lifelong (daily medication, monthly transfusions). | One-time treatment. |
| Curative Potential | Palliative, manages disease progression. Allogeneic HSCT is curative but with significant limitations. | Potentially curative by addressing the genetic root cause. |
| Donor Requirement | No donor for hydroxyurea; matched donor for allogeneic HSCT; universal donor for transfusions. | No donor required for cells (uses patient's own cells); viral vector as delivery vehicle. |
| Immunological Risk | Allosensitization from transfusions; GvHD from allogeneic HSCT. | Minimal risk of rejection (autologous cells); potential for insertional mutagenesis (low). |
Creative Biolabs' Services
Scientists at Creative Biolabs have developed several optimized LVs expressing the human β-AS3 gene under the control of a short promoter and a reduced version of the β-globin LCR. These vectors allow high transgene expression, which is essential for high-titer vector production. These optimized LVs can be potentially used in the clinical trials aimed at establishing the safety and efficacy of HSPCs transduced with LV for gene therapy for SCD.
Service Highlights
- Third-Generation Safety Enhanced LVs: Our carrier is engineered based on advanced third-generation design, incorporating multiple safety modifications to ensure immunogenicity and minimize the risk of insertion mutations.
- Optimized Transgene Expression: Our proprietary approaches for promoter choice, LCR inclusion, and codon optimization ensures high-level, stable and cell type specific expression of the therapeutic gene.
-
High Titer and Purity: Our optimized production and purification scheme results in extremely high titer and purity, both of which are critical for efficient transduction and minimizing in vivo toxicity.
Broad Tropism and Targeted Delivery: While ensuring broad targeting with VSV-G pseudotyping, we specifically evaluated different strategies to augment LV cell specific targeting through ligand receptor interactions, pseudotyping modifications, or tissue-specific promoters.
Frequently Asked Questions
Q: What is a lentiviral vector, and how is it used for SCD gene therapy?
A: A lentiviral vector is a gene delivery vector that is created from a retrovirus (most often HIV-1, with all pathogenic genes removed). They are engineered to safely deliver therapeutic genes (such as anti-sickle beta globin) into target cells. For SCD gene therapy, lentiviral vectors are used to transduce patient's own hematopoietic stem cells in vitro. The genetically modified cells are then returned to the patient, where they engraft into the bone marrow and continuously produce healthy, functional red blood cells.
Q: How does the safety of LV gene therapy compare to that of allogeneic transplantation?
A: LV gene therapy has several advantages over allogeneic transplantation in terms of safety. As an autologous method, there is no risk of graft-versus-host disease, and no need for immunosuppression. The recently developed low-intensity conditioning regimen further reduces surgical risks, by reducing blood toxicity to the greatest extent possible.
Q: What are the potential side effects or safety concerns of lentiviral gene therapy for SCD?
A: While overall it is a safe process, there are some potential issues to be aware of, which include:
a) marrow clearing conditioning: as the patient's bone marrow must be cleared before the modified cell therapy can be delivered, chemotherapy must be used. As with any chemotherapy, there are potential associated side effects including infection, mucositis and organ toxicity.
b) insertion mutation: while modern SIN-LVs are a rare event, there is a theoretical risk of vector integration into the host genome, which could lead to oncogene activation or tumor suppressor gene disruption, and therefore tumorigenesis. This risk is low, but must be monitored for during clinical follow-up.
c) off target effect: While the left ventricle is designed to have as much specificity as possible, off target effects are still possible. Our vector optimization and stringent quality control help to reduce the potential for these issues.
Q: How persistent is lentiviral vector gene expression?
A: A key benefit of lentiviral vectors is their ability to stably integrate genetic material into the host cell genome. This results in long-term, usually lifelong, therapeutic gene expression, especially in long-lived cells such as hematopoietic stem cells. This has been seen in clinical trials, where the therapeutic effects were seen for many years post-treatment.
Q: How does Creative Biolabs ensure quality and safety of its lentiviral vectors?
A: We have the highest industry standards in place for the production of our vectors. Our extensive quality control system covers physical and functional titers, purity, sterility, endotoxin levels and, most importantly, testing for the presence of replication capable lentiviruses (RCLs). Every batch of product is accompanied by an extensive analysis certificate for traceability and compliance.
Get in Touch Today!
With our advanced platforms and experienced technical team, Creative Biolabs is fully equipped to be your one-stop provider for LV development in Sickle Cell Disease (SCD). We offer comprehensive solutions as well as customized services tailored to our clients' specific needs. Please contact us for more information and a detailed quote.
Reference
- Barak M, Hu C, Matthews A, et al. Current and future therapeutics for treating patients with sickle cell disease. Cells, 2024, 13(10): 848. https://doi.org/10.3390/cells11111843 (Distributed under Open Access license CC BY 4.0, without modification.)
