Introduction of Adenovirus as Oncolytic Virus
The therapeutic utilization of viruses against cancer has been renewed during the last two decades. Oncolytic viruses (OVs) are an emerging and effective treatment for many different cancers and have currently been the focus of extensive research to develop their therapeutic potential. OVs replicate and spread within tumor cells, amplifying their cytotoxicity, and reversing the tumor immune suppression at the same time. Among numerous viruses, recombinant adenoviruses (Ads) have been designed to selectively replicate in tumor cells and then tested by intratumoral or systemic administration in clinical trials. Limited efficacy is related to poor tumor targeting, intratumoral spread, and virocentric immune responses. A deeper understanding of the three barriers would be required to produce high-quality oncolytic adenoviruses that, alone or combined with immunotherapy or chemotherapy, may become a powerful tool for oncologists.
Adenoviruses
Adenoviruses are non-enveloped viruses with an icosahedral capsid that comprises a linear double-stranded DNA (dsDNA) of about 36 kb. They belong to the Adenoviridae family, and have been found in all vertebrates, from fishes to humans, displaying species-specificity. Adenoviruses have been classified in serotypes based on the reactivity of antibodies they produce in the hosts, phylogenetic analysis of homologous genes, as well as the total gene content. For example, there're 57 serotypes of the human virus that have been described so far, and these serotypes are divided into 7 subgroups (A to G) according to the cross-reactivity patterns of neutralizing antibodies. Adenoviruses have robust genome insertion capacity that can carry the therapeutic genes of sizes of approximately 7.5 kb, by deleting some viral genes (e.g. E3 gene). The Ads' genome is made of 4 early genes (E1-E4), responsible for virus replication, and 5 late genes (L1-L5), acting as code capsid proteins.
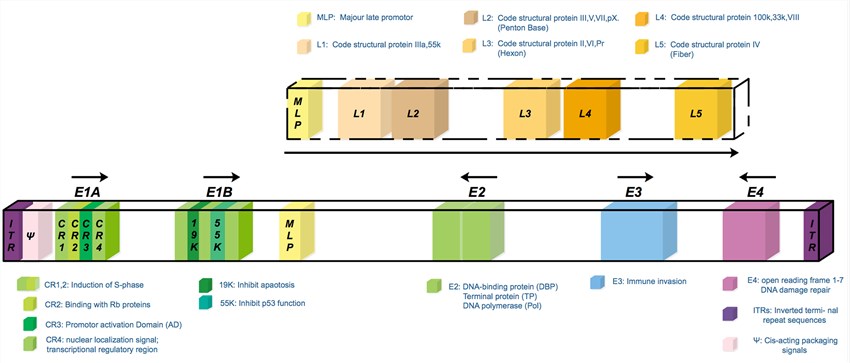 Fig.1 The scheme of the wild type serotype 5 adenovirus genome. (Abudoureyimu, 2019)
Fig.1 The scheme of the wild type serotype 5 adenovirus genome. (Abudoureyimu, 2019)
The Development of Adenovirus-mediated Virotherapy
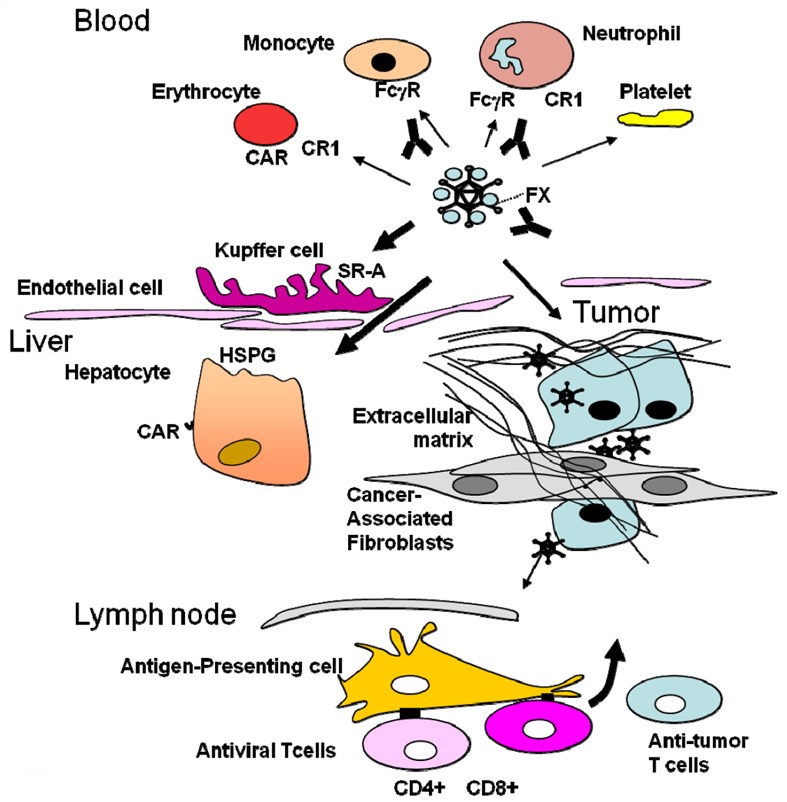 Fig.2 Obstacles to overcome in adenovirus-mediated virotherapy. (Alemany, 2014)
Fig.2 Obstacles to overcome in adenovirus-mediated virotherapy. (Alemany, 2014)
- Adenovirus serotype 5 (AdHu5)
Adenovirus is one of the most frequently studied tools in oncolytic therapy and is extensively applied at present in preclinical and clinical trials. There are several strains of adenovirus and of these, AdHu5 is the most popular used serotype in oncolytic therapy. As the virus is widely exploited, several modifications have been demonstrated to enhance its efficacy and safety.
Typically, oncolytic adenoviruses are developed in terms of human AdHu5. But AdHu5 shows drawbacks of preexisting anti-AdHu5 immunity in most people, and extensive sequestration of Adhu5 by the liver. To address these problems, a novel oncolytic adenovirus AdC7-SP/E1A-ΔE3 has been designed for cancer treatment. This virus was constructed by substituting the E1A promoter with tumor-specific promoter survivin promoter and removing the E3 region by direct cloning approaches on the basis of simian adenovirus serotype 24. AdC7-SP/E1A-ΔE3 has revealed a strong capacity to significantly kill tumor cell lines such as NCI-H508 and Huh7, and repress tumor growth in NCI-H508 and Huh7 xenograft tumor models. Thus, AdC7-SP/E1A-ΔE3 is considered as a promising candidate for liver and colon tumor therapeutics.
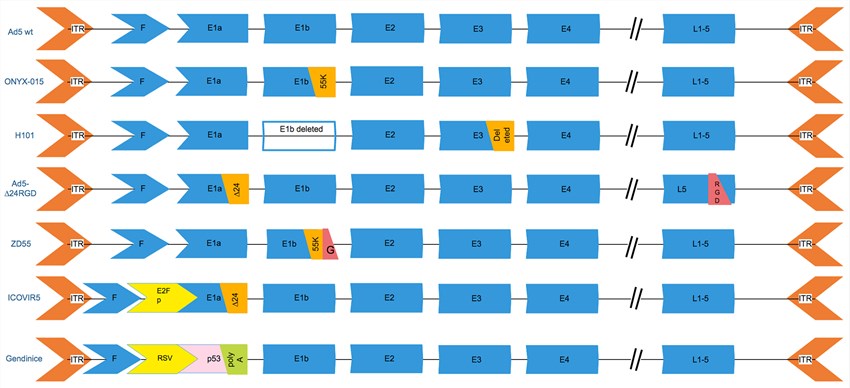 Fig.3 The genetic modifications of common oncolytic adenovirus vectors. (Alemany, 2014)
Fig.3 The genetic modifications of common oncolytic adenovirus vectors. (Alemany, 2014)
- Oncolytic adenovirus in clinical research
It has been proven that the addition of multiple genes to oncolytic adenoviruses could improve their anti-tumor efficacy. For instance, the combination of p53 addition to inhibiting tumor growth with granulocyte-macrophage colony-stimulating factor (GM-CSF) addition to induce the apoptotic pathway generates a synergistic effect which is valid in combating hepatocellular cancer stem cells. This is extremely exciting as it may provide a mechanism to fight with cancer stem cells which are regarded to be integral to cancer recurrence after currently existing treatments.
It is also possible to improve oncolytic adenoviruses by incorporating a short-hairpin RNA (shRNA) which serves to downregulate Dicer enzymes (an endoribonuclease in charge of processing virus-associated RNA). Downregulation of this protein represses the destruction of viral RNA and permits adenoviruses to efficiently replicate and thus increases the efficacy of this kind of OV.
Furthermore, gene silencing methods can also be utilized to downregulate specific oncogenes aiming to suppress tumor growth. For instance, downregulation of EphA3 by inserting siRNA targeting this gene into the genome of an adenovirus whose replication is made conditional under the control of TERTp (increasing specificity for tumor cells) leads to increased expression levels of autophagy through the inhibition of AKT/mTOR pathways. This confers the virus to both repress tumor cell proliferation and clear infected cells.
If you are like to know more about our OncoVirpy™ Platform, please directly send us an e-mail or contact us for your special requests.
References
- Abudoureyimu, M.; et al. Oncolytic adenovirus-a nova for gene-targeted oncolytic viral therapy in HCC. Front Oncol. 2019, 9: 1182.
- Alemany, R. Oncolytic adenoviruses in cancer treatment. Biomedicines. 2014, 2(1): 36-49.
