Introduction of Herpes Simplex Virus as Oncolytic Virus
At present, oncolytic viruses (OVs) have been proposed to be employed as a novel, effective treatment for fatal cancer. Well targeted, they serve the aim of killing tumor cells without eliciting damage to normal cells. In this type of OV drug, human pathogen herpes simplex virus (HSV) is particularly suitable for the cause. Though most viral infection results in an antiviral reaction in the host, HSV has several mechanisms to get out of those responses. Therefore, robust anti-tumor effects can be accomplished by genetic manipulation of HSV genes implicated in this evading mechanism, namely mutations or deletions that modulate its function towards a tumor microenvironment. Recently, oncolytic HSV (oHSV) is broadly used in clinical trials, and there's a great hope that its curative effect will be largely improved by the combination of oHSV with both conventional therapeutics and emerging treatment options.
Herpes Simplex Virus
HSV, a member of the α-herpesviruses subfamily, has many similarities with other viruses, such as pseudorabies virus, varicella-zoster virus, and infectious bovine rhinotracheitis virus. This virus comprises double-stranded DNA (dsDNA) genomes of ≥120 kb, responsible for encoding 70 or more genes. There're two existing variants of HSV, including HSV type 1 (HSV-1) and HSV type 2 (HSV-2). Among these two subtypes, HSV-1 has been widely investigated in cancer oncolytic viral therapy. HSV-1 vectors are divided into amplicon, replication-defective vectors, and conditionally replicating vectors. Replication defective vectors consist of transgene expression cassettes, integrated into the viral genome with one or more crucial viral genes deleted. Such type of HSV-1 vectors displays a high expression level of transgene products, while being incapable to multiply within cells which otherwise don't complement the missing viral function in trans. Today, lysotype HSV is the first virus to be constructed as a recombinant OV therapeutic vector, and the first OV to combat cancer.
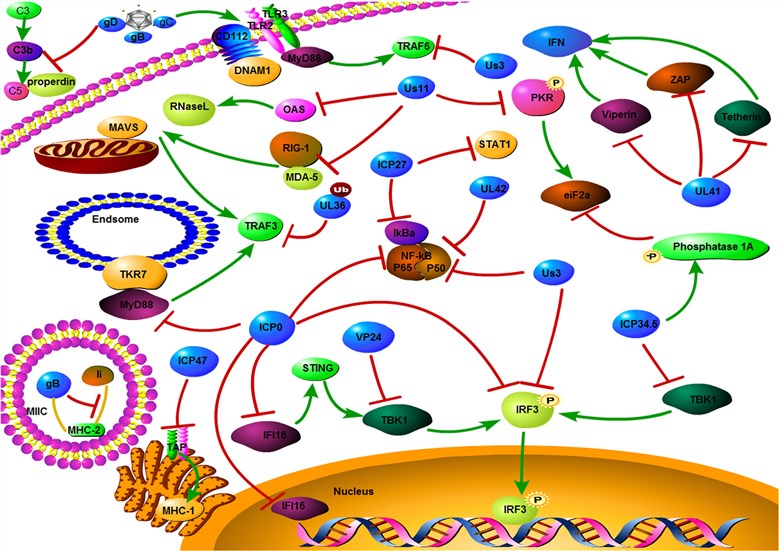 Fig.1 Mechanism of HSV evades host immune responses. (Ma, 2018)
Fig.1 Mechanism of HSV evades host immune responses. (Ma, 2018)
Advances in Oncolytic Herpes Simplex Viral Therapy
HSV is a neurotropic DNA virus that has been one of the most prevalently studied therapeutics and used OV. This virus has some capability to escape the host's immune response. Deletion or mutation of genes that are involved in HSV's escape through its host' immune defense will inhibit its replication in normal cells. In general, the tumor microenvironment is in an immunosuppressive state, which possibly permits the virus' entry and replication, which in turn ultimately leads to the death of tumor cells. As well, HSV could reverse the immunosuppression of tumor microenvironment, enhance the tumor immunogenicity, improve the infiltration of inflammatory cells, as well as play a valid anti-tumor effect. As a cytolytic virus, oHSV shows the following highlights:
- HSV replicates quickly in cells and has an ability to infect many types of cancer cells;
- HSV possesses a large genome, which can be easily modified and inserted with several additional transgenes;
- HSV can be prevented with antiviral medicines when the dosage starts to impose a threat to the patients' lives;
- Modifying the glycoproteins of HSV can promote the targeting of tumor cells.
- Advances in clinical development of oHSV
- Systemic delivery of oncolytic HSV to tumor cells by retargeting
Genetic engineering of the OV genome to increase its anti-cancer selectivity has opened the new era of OV therapy. This can be traced to an early publication, in which thymidine kinase-deleted HSV-1 moderated the neurovirulence in a murine glioblastoma model. Two current Phase I/II clinical trials demonstrate the efficacy of single-agent in oncolytic viral therapy, backed by more than one report. One of the most convincing evidence of the ability of OVs is to significantly augment anticancer immunity. The intratumoral injection is provided by a trial that takes advantage of a second-generation oHSV-1. This drug is intratumorally administered, resulting in total regression of uninjected and injected lesions of 8/50 patients treated with metastatic malignant melanoma.
An obvious drawback of systemic OV delivery is off-target viral replication in patients whose immunity is injured. To deal with the off-target problem, one effective method adopted is retargeting the virus infectivity to obtain infection of the target cell selectively. Retargeting is done by several strategies, such as modifying present viral glycoproteins to contain single chain antibodies (scFv) or peptide ligands that are coupled to the required receptor, utilizing soluble adapters which identify both the OV and an exclusive receptor on the target cell or inserting glycoproteins with different host range from other viruses. The process of systemic delivery of oncolytic HSV-1 to the tumor cells by retargeting has been described, including eliminating the ingress by the native gD receptors of HSV simultaneously attaining infections similar to the ones obtained with wt-HSV on subjected cells. Alongside HSV-1, HSV-2 has also exhibited its promise as an OV. For instance, HSV-2 with a deletion of ICP10 to enhance selectivity was engineered and thought of even more effective than HSV-1 for the treatment of metastatic ovarian cancer in murine models. Based on this initial success, more research is being conducted to promote HSV-2 as a powerful OV therapy.
Reference
- Ma, W.; et al. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19(1): 40. Distributed under Open Access license CC BY 4.0, without modification.
