Introduction of Parvovirus as Oncolytic Virus
Oncolytic virotherapy of cancer is among innovative approaches being under development and particularly valuable for targeting unsolved tumors, which are resistant to traditional treatments. At present, more than a dozen viruses, derived from 9 different virus families, are being analyzed within the frames of multiple clinical studies in cancerous people. Accumulating preclinical evidence demonstrating that parvovirus (Parvovirus H-1, H-1PV) can eliminate tumor cells that resist traditional anticancer treatments and to accomplish a complete cure of different human tumors in animal models argues for its involvement in the arsenal of oncolytic viruses (OVs). Oncolytic parvovirus safe administration to humans is dependent on the intrinsic preference of these agents for tumor selectivity. Conclusively, existing data and its highly favorable safety profile allow the translation of parvovirus' preclinical studies into a Phase I/IIa clinical trials currently in progress.
Basic Characteristics of Parvovirus
The Parvoviridae family contains 134 viruses that can infect a wide range of hosts. They're featured by an icosahedral capsid of ~25 nm in diameter with a linear, single-stranded DNA genome of ~5000 nucleotides. The family is classified into 2 subfamilies, Parvovirinae and Densovirinae, members of which respectively invade vertebrates and arthropods. Parvoviruses (PVs) are a type of small non-enveloped icosahedral particles. When they infect a permissive cell, the viruses come into a lytic cycle whose major replication and assembly steps occur in the nucleus and which generally result in the death of target cells. According to their type and physiological state, PV-infected cells may die in the following ways, such as an oncotic pathway, classical apoptosis, or lysosomal mechanism. In particular, several PVs are regarded as a powerful tool for anticancer applications owing to their biology, which is closely interrelated with the biology of host organisms. PVs are often named "viruses in search of a disease".
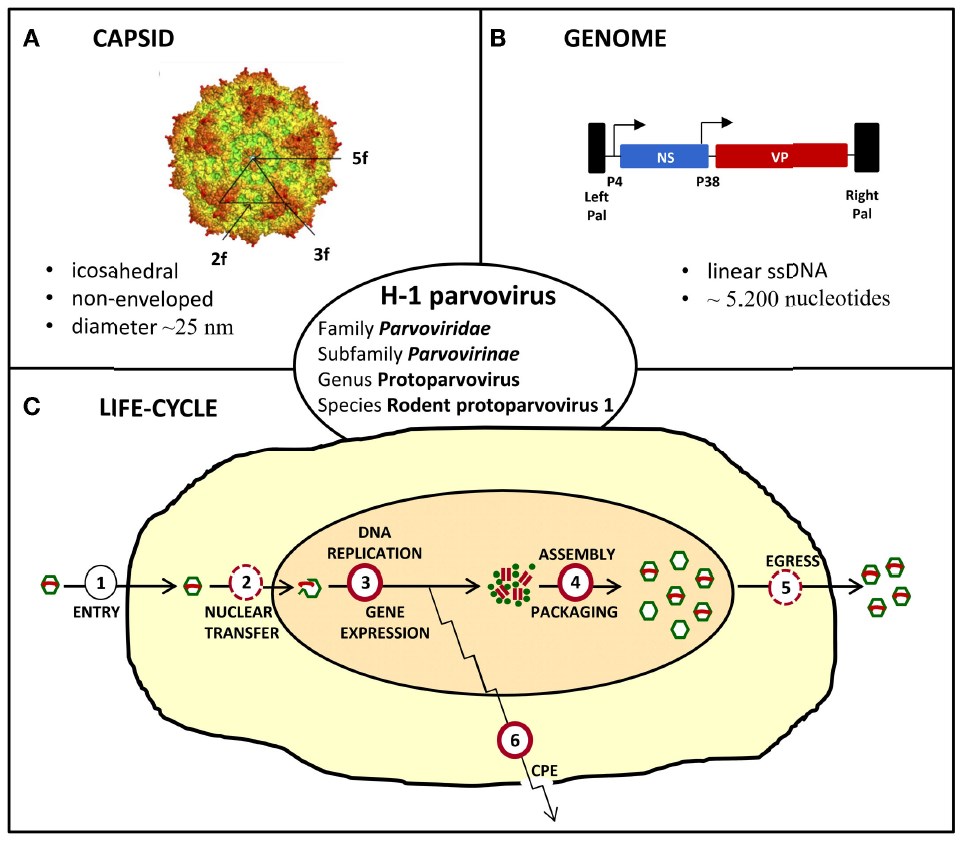 Fig.1 Characteristics of H-1 parvovirus. (Angelova, 2015)
Fig.1 Characteristics of H-1 parvovirus. (Angelova, 2015)
Parvoviruses as Oncolytic Agents
Advances in animal cells demonstrated the capacity of PVs to selectively kill transformed cells and suppress their tumor-forming ability in vivo. Accumulated evidence has revealed that some members of the Parvoviridae family, especially the rodent protoparvovirus H-1PV, the minute virus of mice shows inherent anticancer activity without pathogenic to humans. Extended observations found that H-1PV also can efficiently eliminate various human tumor cells in culture, and leads to the regression of human mammary and cervical carcinoma implants in immunocompromised mice. These investigations have laid the foundations for the launch of a first Phase I/IIa clinical trial, in which the mice H-1PV is undergoing evaluation for its safety and efficacy in patients with a brain tumor.
- The assessment of oncolytic parvovirotherapy in a malignant brain tumor model
- Assessment of oncolytic parvovirotherapy in a non-Hodgkin lymphoma model
Patients with glioblastoma multiforme (GBM) have a poor prognosis with a median survival of 12-16 months after diagnosis. The last decade's progress in diagnostics, surgery, and chemoradiotherapy hasn't improved patient survival greatly. Oncolytic virotherapy is likely to be an alternative therapeutic concept and H-1PV makes this virus a promising agent for such an approach because of its oncotropism and selective cytotoxicity. Rat and human glioblastoma, gliosarcoma cell lines, and early passage human glioma cells have proven sensitive to the killing effect of H-1PV at relatively low multiplicities of infection, whilst healthy brain cells exhibit resistance to viral cytopathic effects. In laboratory testing, intracerebral injection of rat H-1PV doesn't induce any damage to the brain or other cells. These facts promote the studies of H-1PV-based virotherapy preclinically in an immunocompetent rat glioma model. For now, H-1PV therapy has allowed significantly improved survival and in most cases, even complete remission.
Burkitt's lymphoma (BL) is a very fast-growing non-Hodgkin lymphoma, which has been tested for its sensitivity to H-1PV. Both drugs cause short- or long-term side effects and the selection of resistant tumor cell variants. This assist scientists to perform preclinical studies on the potential benefits of oncolytic H-1PV against this type of disease. H-1PV can exert direct oncolytic effects not only in vitro (BL-derived cell lines) but also in vivo (a BL xenograft model). BL-derived cell lines seem to be greatly sensitive to H-1PV-induced.
Reference
- Angelova, A.L.; et al. Tumor selectivity of oncolytic parvoviruses: from in vitro and animal models to cancer patients. Front Bioeng Biotechnol. 2015, 3: 55. Distributed under Open Access license CC BY 4.0, without modification.
