Introduction How Can We Help? Deliverables Key Benefits Related Products FAQs Contact
Are you currently facing challenges related to systemic toxicity, high manufacturing costs, or complex pharmacokinetics in developing targeted therapeutics for chronic conditions? Creative Biolabs' specialized ApDCs Development service helps you rapidly create highly selective, chemically stable drug conjugates through our advanced SELEX and synthetic conjugation platform, delivering drugs precisely to the site of cardiovascular or metabolic pathology.
Introduction of ApDCs Development for Cardiovascular and Metabolic Disorders
ApDCs, or Aptamer-Drug Conjugates, represent a transformative approach in precision medicine. They combine the unparalleled specificity of nucleic acid ligands (aptamers) with potent therapeutic payloads, offering a targeted solution that minimizes the systemic exposure plaguing current treatments for chronic disorders.
What Is Our Service?
Our service provides ApDC, utilizing a short, single-stranded DNA or RNA aptamer as a sophisticated homing device chemically linked to a therapeutic payload (e.g., a small molecule drug, an anti-inflammatory oligonucleotide, or a metabolic regulator), customized for Cardiovascular and Metabolic Disorders.
Application Scenarios
-
Anti-Thrombosis: Developing ApDCs that target clotting factors, to deliver highly potent anticoagulants directly to the site of vascular injury, significantly reducing the risk of systemic bleeding compared to broad-spectrum anticoagulants.
-
Metabolic Disease: Engineering ApDCs to target specific receptors on activated cells to deliver anti-fibrotic or insulin-sensitizing payloads, addressing core pathology with high tissue selectivity.
-
Atherosclerosis Treatment: Targeting receptors specifically expressed on activated endothelial cells within atherosclerotic plaques to deliver lipid-lowering or anti-inflammatory agents, stabilizing lesions and preventing rupture.
Why Choose Us?
Creative Biolabs leverages the inherent biological and chemical advantages of aptamers to create superior therapeutic agents.
|
Pain Points
|
Benefit Created by Creative Biolabs
|
|
High Manufacturing Cost/Variability
|
Chemical Synthesis: Aptamers are synthesized rapidly and affordably at scale using automated chemistry, ensuring zero batch-to-batch variation.
|
|
Systemic Toxicity/Off-Target Effects
|
Superior Selectivity: Aptamers are highly specific ligands that guide the payload precisely via Receptor-Mediated Endocytosis to disease tissue.
|
|
Short Half-Life in Chronic Dosing
|
Proprietary PEGylation & Modification: Site-specific chemical modification ensures robust stability against nucleases and extended circulation time necessary for chronic disease management.
|
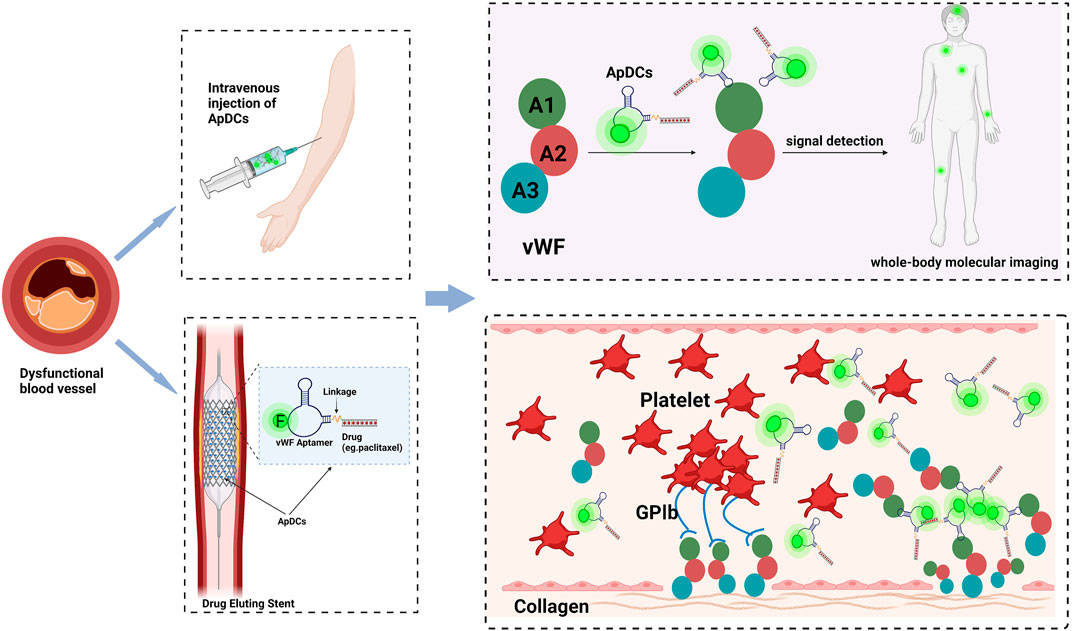 Fig.1 ApDC applications in cardiovascular disease for future diagnostic and therapeutic integration.1
Fig.1 ApDC applications in cardiovascular disease for future diagnostic and therapeutic integration.1
How Creative Biolabs' ApDC Development Can Assist Your Project?
Creative Biolabs provides an end-to-end ApDC development pipeline, meticulously designed for chronic indication therapeutics, transitioning seamlessly from target validation to preclinical assessment.
Workflow of ApDC Development
-
Required Starting Materials
-
Target Protein/Receptor
-
Therapeutic Payload Chemistry
-
Disease Model Data
-
Target Validation & SELEX Screening
-
Aptamer Optimization & Stabilization
-
Linker & Conjugation Chemistry
-
In Vitro Efficacy & Selectivity Validation
-
PK and PD Assessment
Tap into Our Knowledge – Schedule Your Consultation Now!
Deliverables
Creative Biolabs ensures transparent and complete delivery of all required technical and analytical outputs at each stage of the ApDC development lifecycle.
-
Final ApDC Product
-
Binding and Selectivity Data: Raw and analyzed data confirming aptamer affinity for the target versus non-target proteins.
-
Stability and Modification Report: Documentation on the chemical modifications used and the ApDC's stability profile under various physiological conditions.
-
Cellular Uptake Kinetics: Quantitative data on the ApDC's uptake efficiency and concentration within the target cell population in vitro.
-
Linker Cleavage and Drug Release Profile: In vitro data confirming the stability of the linker in serum and its controlled release profile under relevant trigger conditions.
-
Full Lab Report and Methodology: Detailed SOPs and protocols used for SELEX, synthesis, and all analytical assays.
Key Benefits
Creative Biolabs' dedicated ApDC platform offers unique advantages that substantially de-risk and accelerate the path to a clinically viable therapeutic for chronic diseases.
Unmatched Pharmacokinetic Tuning
Through precise PEGylation and stabilizing modifications, we can tailor the in vivo half-life of the ApDC to match the required therapeutic window of chronic cardiovascular or metabolic disorders, avoiding the rapid renal clearance typical of unmodified oligonucleotides.
Low Immunogenic Profile
Unlike large protein-based conjugates, the nucleic acid backbone of the aptamer carries a low intrinsic risk of eliciting an immune response, resulting in a favorable safety profile essential for long-term dosing.
Versatile Payload Integration
Our expertise includes coupling a wide range of payloads—from classic small molecules to novel metabolic regulators—via diverse and intelligent linker chemistries, providing flexibility to address heterogeneous disease targets.
Targeting Complexity
Our SELEX technology can generate aptamers against challenging targets, including cell-surface receptors that are difficult to bind with antibodies or complex protein aggregates present in plaques, broadening the treatable target space.
Discover the Creative Biolabs Edge – Request a Quote Now!
Related Products
Frequently Asked Questions
Q1: Since ADCs are more established, why should we switch our focus to ApDCs for a cardiovascular target?
A: ApDCs offer crucial advantages for chronic disease. They are chemically synthesized, meaning greater purity, lower cost, and absolute consistency (no batch variation) compared to ADCs. Furthermore, their smaller size may offer superior tissue penetration into structures like atherosclerotic plaques or dense metabolic tissues, which is often challenging for large antibodies.
Q2: What level of targeting specificity can I expect, and how do you confirm precise drug delivery?
A: Aptamers are selected for extremely high affinity and specificity through our rigorous SELEX platform. We confirm this specificity in vitro using sophisticated biophysical assays. For drug delivery confirmation, we can integrate fluorescent or imaging moieties into the ApDC to visually track and quantify its accumulation in the target tissue in preclinical models.
Q3: What is the typical lead time to move from a validated target to an optimized ApDC candidate ready for preclinical testing?
A: Our specialized workflow, which includes parallel optimization of the aptamer and linker chemistry, allows for an efficient timeline. Depending on whether a de novo SELEX campaign is needed, we typically deliver an optimized, purified ApDC candidate with comprehensive in vitro and preliminary in vivo PK data within 18 to 30 weeks.
Contact Us
Creative Biolabs' ApDCs Development service is uniquely positioned to overcome the limitations of conventional therapeutics in chronic cardiovascular and metabolic disorders. By merging the chemical robustness of aptamers with precision delivery, we enable our partners to develop safer, more effective, and commercially scalable drugs that redefine patient care. Contact us to discuss the details.
Reference
-
Chen, Xinyuan, et al. "Aptamer-based applications for cardiovascular disease." Frontiers in bioengineering and biotechnology 10 (2022): 1002285. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.3389/fbioe.2022.1002285.
For Research Use Only.
Related Sections:

 Fig.1 ApDC applications in cardiovascular disease for future diagnostic and therapeutic integration.1
Fig.1 ApDC applications in cardiovascular disease for future diagnostic and therapeutic integration.1