Introduction How Can We Help? Deliverables Key Benefits Related Products FAQs Contact
Are you currently facing systemic toxicities from broad-spectrum immunosuppressants, off-target effects in complex autoimmune diseases, or challenges in obtaining high-specificity delivery ligands? Our Creative Biolabs ApDCs Development Service helps you create highly selective immunomodulators and potent anti-inflammatory agents through advanced SELEX optimization, proprietary conjugation chemistry, and focused delivery to disease-specific immune cell populations.
Introduction of ApDCs for Immunomodulation
Aptamer-Drug Conjugates (ApDCs) represent the next-generation platform for targeted therapy, leveraging short, single-stranded nucleic acids (aptamers) to deliver potent immunomodulatory or anti-inflammatory payloads directly to the disease site. Unlike large, protein-based antibodies, aptamers fold into unique three-dimensional structures, enabling them to bind to target molecules with high affinity and selectivity, including surface receptors on specific immune cell subsets like activated T-cells or plasmacytoid dendritic cells (pDCs). This inherent biological advantage allows for a precision approach critical in mitigating the side effects typically associated with systemic immunosuppression or aggressive immune activation.
What Is Our Service?
Our service provides end-to-end development, from initial target identification and aptamer selection to the final chemical conjugation and preclinical validation of a stable, functional ApDC. The goal is to maximize the therapeutic index by selectively concentrating the anti-inflammatory or immunomodulatory payload at the site of pathology. Application Scenarios:
-
Autoimmune Disease Treatment: Targeted delivery of corticosteroids or immunosuppressants to tissue-infiltrating immune cells to suppress inflammation locally.
-
Cytokine Receptor Antagonism: Development of aptamers that act as antagonists against inflammatory cytokines or their receptors, conjugated with a molecule to enhance cellular uptake and persistence.
-
Immune Checkpoint Modulation: Precision delivery of agonists or antagonists to specific immune populations to fine-tune the immune response in inflammatory bowel diseases (IBD) or chronic inflammation.
Why Choose Us?
Creative Biolabs creates exceptional value by directly addressing the core challenges of immunomodulatory drug development. We convert high systemic toxicity and manufacturing complexity into precision and stability.
|
Pain Points
|
Benefit Created by Creative Biolabs
|
|
Systemic Toxicity of Immunosuppressants
|
Reduced Off-Target Effects: ApDCs selectively deliver the therapeutic payload to the targeted cell population, confining activity to the inflamed tissue and dramatically reducing systemic exposure and associated toxicities.
|
|
High Cost & Complexity of Biologics
|
Chemical Synthesis and Scalability: Aptamers are chemically synthesized, ensuring high batch-to-batch consistency and significantly lowering large-scale manufacturing costs compared to antibody production.
|
|
Poor Tissue Penetration
|
Enhanced Efficacy at Target Site: The smaller size of ApDCs allows for deeper penetration into dense, inflamed tissues and tumor microenvironments than large antibodies, improving local bioavailability of the drug.
|
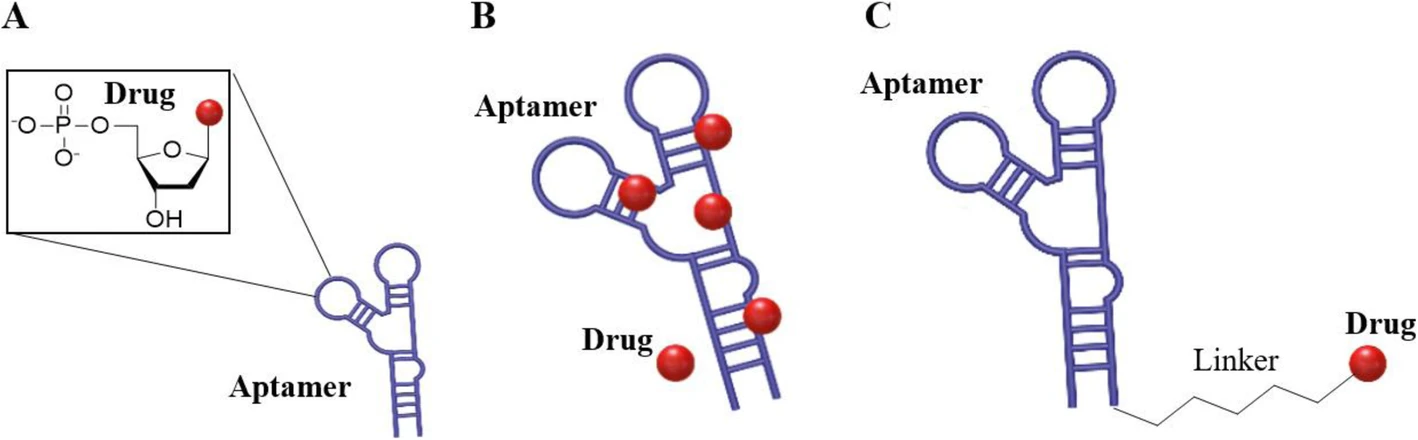 Fig.1 Schematic diagram of ApDC.1
Fig.1 Schematic diagram of ApDC.1
How Creative Biolabs' ApDC Development Can Assist Your Project?
Our comprehensive and detailed workflow ensures every aspect of ApDC design, from aptamer binding affinity to linker stability, is optimized for your immunomodulatory or anti-inflammatory application.
Workflow of ApDC Development
-
Required Starting Materials
-
Specific Target Information
-
Payload Characteristics
-
Indication Requirements
-
Target Validation & SELEX Strategy Design
-
Aptamer Selection (Optimized SELEX)
-
Chemical Modification & Optimization
-
Conjugation Chemistry & Linker Selection
-
Preclinical Efficacy & Specificity Screening
Discover Our Proficiency – Arrange Your Consultation Now!
Deliverables
Upon completion of the ApDC Development Service, Creative Biolabs provides a complete data package to ensure seamless transition to the next stage of your drug development pipeline:
-
Final ApDC Construct: Optimized, purified Aptamer-Drug Conjugate material, ready for advanced studies.
-
Detailed Lab Report: Documentation covering all selection and synthesis stages, including purification protocols and quality control data.
-
Binding Affinity and Specificity Data: Raw data and model annotations demonstrating affinity and selectivity against non-target cells.
-
In Vivo Efficacy and Toxicity Reports: Data on therapeutic efficacy in relevant animal models, pharmacokinetics (PK), half-life measurements, and a preliminary assessment of systemic toxicity.
Key Benefits
Leveraging our proprietary SELEX and conjugation platforms provides distinct advantages in creating superior immunomodulatory therapeutics.
Superior Targeting Versatility
Aptamers can be selected against non-protein targets, such as small molecules or entire living cells (Cell-SELEX), enabling the targeting of biomarkers that are inaccessible to traditional antibody development.
Scalability and Cost-Effectiveness
Aptamers are synthesized via rapid, inexpensive chemical synthesis, ensuring a steady, high-quality, and cost-effective supply, which is a significant logistical benefit for preclinical and clinical scale-up.
Fast Tissue Penetration
The small size (typically 10-20 kDa) of ApDCs allows them to navigate dense inflammatory matrices and achieve high local concentrations, leading to a faster and more potent local therapeutic effect.
High Batch Consistency
Due to the non-biological nature of the synthesis, our ApDCs exhibit negligible batch-to-batch variation, accelerating regulatory processes and ensuring reliable, reproducible results throughout your project lifecycle.
Tap into the Creative Biolabs Advantage – Obtain Your Quote Today!
Related Products
Frequently Asked Questions
Q: How does the ApDC platform ensure reduced systemic toxicity compared to conventional drugs?
A: Our ApDCs achieve highly localized drug delivery. The aptamer acts as a molecular zip code, binding almost exclusively to a biomarker overexpressed on the pathological cell or tissue. This concentration of the payload at the disease site means a much lower systemic drug dose is required to achieve efficacy, fundamentally improving the drug's therapeutic index and safety profile.
Q: What materials or information do I need to start an ApDC project, and how long does the process take?
A: To begin, we require information on your specific disease target (e.g., protein sequence, cell line, or known inflammatory pathway) and the therapeutic agent you wish to conjugate. The typical full development cycle, including aptamer selection, optimization, and initial in vivo testing, ranges from 18 to 24 weeks. This timeline ensures we generate sufficient binding and stability data before moving to advanced conjugation.
Q: Can Creative Biolabs develop ApDCs for targets without known cell surface markers?
A: Yes, our advanced Cell-SELEX techniques allow us to select aptamers against entire living cells, even if the specific target biomarker is unknown. This approach is highly effective for identifying unique molecular signatures in diseased cell populations, providing a powerful advantage for targeting complex, heterogeneous inflammatory diseases.
Contact Us
Creative Biolabs is dedicated to advancing precision medicine through the development of highly specific ApDCs for immunomodulatory and anti-inflammatory applications. Our expertise in targeted delivery reduces systemic toxicity and accelerates the path to clinic for next-generation therapeutics. To discuss your specific research needs or to begin the ApDC development process, please reach out to our scientific team.
Reference
-
Kim, Do-Hun, et al. "Design and clinical developments of aptamer-drug conjugates for targeted cancer therapy." Biomaterials Research 25.1 (2021): 42. Distributed under Open Access license CC BY 4.0, without modification. DOI: https://doi.org/10.1186/s40824-021-00244-4.
For Research Use Only.
Related Sections:

 Fig.1 Schematic diagram of ApDC.1
Fig.1 Schematic diagram of ApDC.1