Product List Background C1 Inhibitor Functional Service
Background
C1 inhibitor (C1 INH), a member of the serpin family of protease inhibitors, has a similar structure and mechanism of protease inactivation to other members of the family. For protease inactivation, the protease recognizes the reactive center loop of serpins, which is displayed above the surface of the molecule. Cleavage of the peptide bond (P1-P1’) at the reactive center induces a molecular rearrangement and leads to covalent bond generation between the P1 residue of the inhibitor and the active site serine of the protease. C1 INH inactivates many proteases such as contact system proteases (factor XII, plasma kallikrein), the fibrinolytic proteases (plasmin, tissue plasminogen activator), complement system proteases (C1r, C1s, MASP2), and an intrinsic coagulation protease (factor XI).
Indeed, the major activity of C1 INH is protease inhibition. Nevertheless, a variety of studies have described the non-covalent interaction of C1 inhibitor with many other substances, including the complement component C3, gram-negative endotoxin, extracellular matrix components, endothelial cells, and leukocytes, as well as several infectious agents. It has proven that these interactions do not seem to depend upon protease inhibitor function, at least in some cases, to have potentially essential functional consequences.
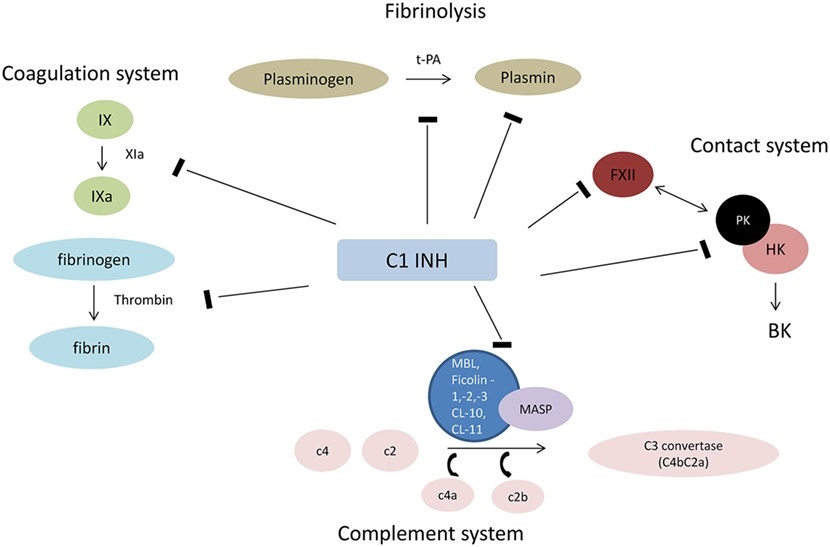 Fig.1 Interaction of C1 INH with plasmatic cascades.1, 3
Fig.1 Interaction of C1 INH with plasmatic cascades.1, 3
C1 Inhibitor Functional Service
Creative Biolabs provides a comprehensive range of C1 Inhibitor-related products, encompassing anti-C1 Inhibitor monoclonal/polyclonal Antibodies, C1 Inhibitor ELISA Assay Kits, and C1 Inhibitor Proteins, which are highly specific for detecting and quantifying C1 Inhibitor activity and concentration, offering reliable tools for inflammation, coagulation, and complement activation research. Our C1 Inhibitor proteins are produced with high purity, and our antibodies provide exceptional sensitivity and specificity, ideal for applications in immunodetection, functional assays, and therapeutic research.
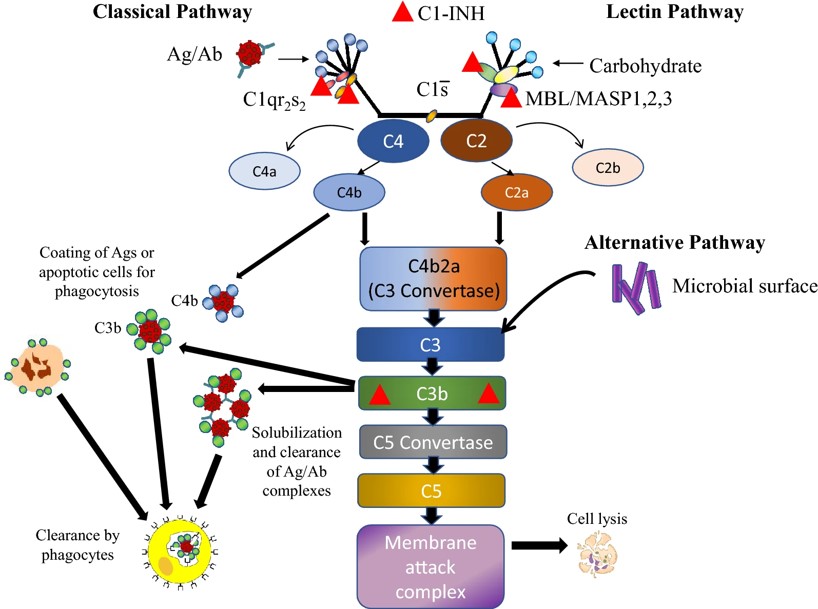 Fig.2 Biological function of C1 INH in the classical and lectin complement pathways.2,3
Fig.2 Biological function of C1 INH in the classical and lectin complement pathways.2,3
Functional testing of the C1 inhibitor is essential to assess its activity and identify potential deficiencies or dysfunctions. Various techniques can be employed for C1 Inhibitor functional testing, including chromogenic substrate assays, amidolytic assays, and immunological assays. ELISA-based assays to quantify C1 Inhibitor activity, flow cytometry for assessing complement activation, and functional inhibition assays using C1 Inhibitor proteins to evaluate their impact on C1 complex activity.
In one study, functional testing of C1 Inhibitor proteins has revealed a significant reduction in C1 activation, confirming its key role in controlling inflammatory responses and offering insights for therapeutic targeting in diseases such as hereditary angioedema. By combining these techniques with our high-quality C1 Inhibitor products, researchers can gain valuable insights into the functional status of C1 Inhibitor and its role in various diseases.
Creative Biolabs provides C1 Inhibitor-functional services, including custom assay development, functional testing, and data analysis, ensuring the highest service quality and accurate, reproducible results.
References
-
Panagiotou, Anneza, Marten Trendelenburg, and Michael Osthoff. "The lectin pathway of complement in myocardial ischemia/reperfusion injury—review of its significance and the potential impact of therapeutic interference by C1 esterase inhibitor." Frontiers in immunology 9 (2018): 1151.
-
Levy, Donald, et al. "Co-occurrence between C1 esterase inhibitor deficiency and autoimmune disease: a systematic literature review." Allergy, Asthma & Clinical Immunology 16 (2020): 1-8.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig.1 Interaction of C1 INH with plasmatic cascades.1, 3
Fig.1 Interaction of C1 INH with plasmatic cascades.1, 3
 Fig.2 Biological function of C1 INH in the classical and lectin complement pathways.2,3
Fig.2 Biological function of C1 INH in the classical and lectin complement pathways.2,3
