Product List Background MBL1 Functional Service
Background
The complement lectin pathway plays a significant role in recognizing and clearing the pathogenic microbes before adaptive immunity. The complement lectin pathway is similar in structure to the classical pathway, except that activation or recognition pattern is different. The activation of the complement lectin pathway is mediated by pattern recognition proteins, including mannan-binding lectins (MBLs), ficolin-1, ficolin-2, ficolin-3, and collectin-11.
MBLs, also known as mannan-binding proteins (MBPs), are large plasma glycoproteins belonging to the collectin family since they all contain collagenous regions and lectin domains. In humans, only one MBL form has been identified, whereas two functional MBL isoforms (MBL1 and MBL2) have been isolated rodent serum. Mouse MBL1 or MBP-A is a polypeptide chain composed of 239 amino acid residues, containing four domains, a cysteine-rich domain at the N-terminus, a collagen-like domain, an α-helical neck region, and a carbohydrate-recognition domain at the C-terminus. Similar to human MBL, mouse MBL1 comprises 21 amino acid residues including 3 cysteine residues at the N-terminal region, which is involved in the oligomerization. Rat MBL1 usually forms a hexamer of subunits composed of three identical polypeptide chains.
MBL1, like complement C1q, forms a complex with MBL-associated serum proteases (MASPs) in a Ca2+ dependent manner. Upon MBL1 bind to the specific carbohydrates (such as mannose, fucose, and N-acetylglucosamine sugar motifs) on the surface of a variety of microorganisms, the MASPs become activated to cleave both C4 and C2, producing C3 convertase (C4b2a) and activating the complement lectin pathway. MBL1 also can bind to late apoptotic cells, apoptotic vesicles, and necrotic cells, but not early apoptotic cells, to promote their uptake and clearance by macrophage. MBL1, as well as MBL2, have been demonstrated to associate with immunodeficiencies, brain ischemia/reperfusion injury, and rheumatic heart disease.
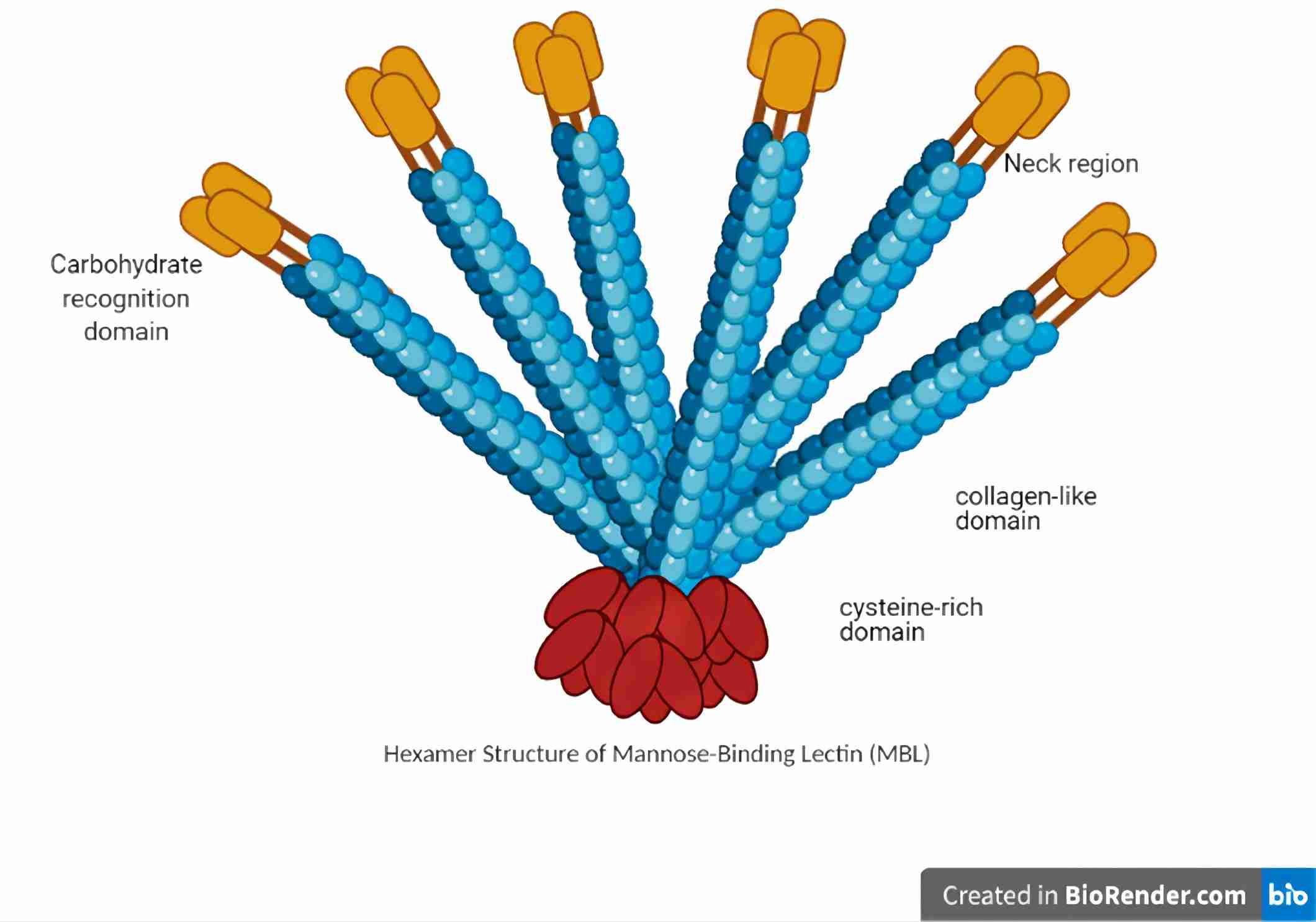 Fig. 1 Schematic diagram of MBL structure.1, 3
Fig. 1 Schematic diagram of MBL structure.1, 3
MBL1 Functional Service
Creative Biolabs supplies an extensive array of MBL1-associated offerings, including ELISA Assay Kits, anti-MBL1 Antibodies, and human complement MBL1 Proteins. These tools proficiently identify and monitor the interaction between antibody regions and the human MBL1 protein, thereby serving a critical function in disease therapy-oriented research.
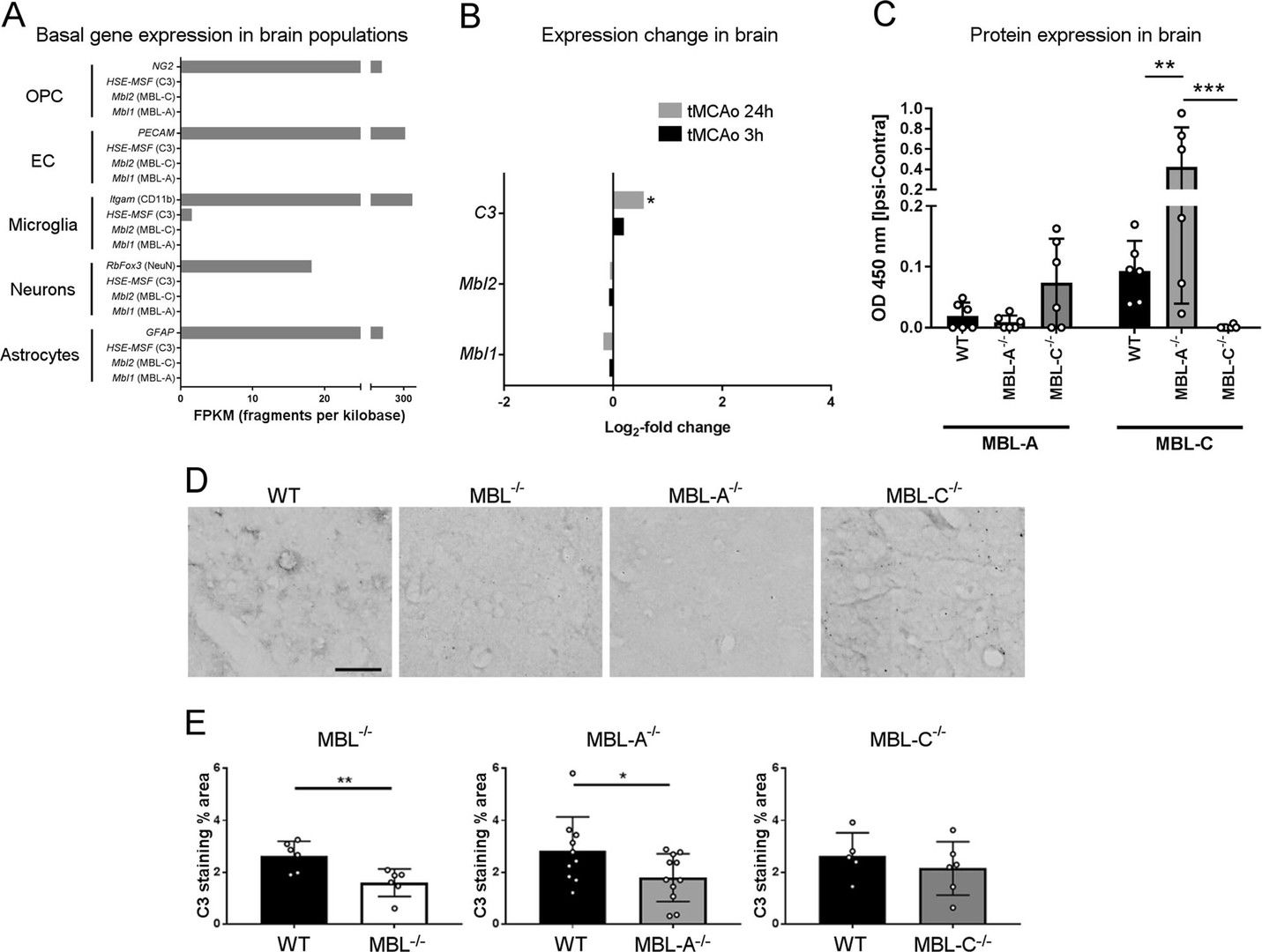 Fig.2 Expression patterns of MBL isoforms and activation of the complement cascade in the brain after ischemia/reperfusion in wild type, MBL knockout, MBL-A deficient, and MBL-C deficient mice.2, 3
Fig.2 Expression patterns of MBL isoforms and activation of the complement cascade in the brain after ischemia/reperfusion in wild type, MBL knockout, MBL-A deficient, and MBL-C deficient mice.2, 3
Clinical investigations and animal models reveal the detrimental impact of MBL in ischemic stroke. Rodents contain two isoforms, MBL-A and MBL-C, with unclear roles. To translate preclinical findings into clinical applications, researchers explored each isoform’s contribution to cerebral ischemia. In experiments with mice lacking both or one MBL isoform under transient middle cerebral artery occlusion, showed in ELISA results, double depletion resulted in reduced neurological deficits and lesion size compared to controls. Single depletion mice experienced smaller or no lesions without behavioral issues. Mbl1 and Mbl2 gene activations were similar post-occlusion, but Mbl1 was activated earlier in WT mice. The depletion study underscored MBL-A’s pivotal role in ischemic injury due to its earlier and more efficient complement activation.
Creative Biolabs offers a suite of MBL1-related services, including detecting MBL1 binding and supplementary functional analyses, designed to support our esteemed clients in clinical and research settings.
References
-
Idowu, Peter A., et al. "Activity of mannose-binding lectin on bacterial-infected chickens—a review." Animals 11.3 (2021): 787.
-
Neglia, Laura, et al. "Specific contribution of mannose-binding lectin murine isoforms to brain ischemia/reperfusion injury." Cellular & Molecular Immunology 17.3 (2020): 218-226.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig. 1 Schematic diagram of MBL structure.1, 3
Fig. 1 Schematic diagram of MBL structure.1, 3
 Fig.2 Expression patterns of MBL isoforms and activation of the complement cascade in the brain after ischemia/reperfusion in wild type, MBL knockout, MBL-A deficient, and MBL-C deficient mice.2, 3
Fig.2 Expression patterns of MBL isoforms and activation of the complement cascade in the brain after ischemia/reperfusion in wild type, MBL knockout, MBL-A deficient, and MBL-C deficient mice.2, 3
