Safety & Tumor Selectivity Enhancement Services
Introduction
Many encounter challenges in developing highly specific, safe, and efficacious cancer therapies. Our Advanced Oncolytic Adenovirus Engineering Service addresses this by leveraging cutting-edge viral engineering and modification strategies to deliver potent, safe oncolytic therapies. The service provides precisely engineered oncolytic adenoviruses with enhanced tumor selectivity and superior safety profiles, directly yielding more effective and tolerable therapeutic candidates. Creative Biolabs prioritizes optimizing vector performance to minimize off-target effects, reduce host immunogenicity, and accelerate preclinical and clinical development timelines, ensuring your therapies achieve robust efficacy while adhering to strict safety standards.
[Discover How We Can Help - Request a Consultation]
Enhancing Safety and Tumor Selectivity
Oncolytic adenoviruses (Ads) are a frontier in cancer therapy, selectively infecting/destroying cancer cells. Enhancing tumor selectivity and safety via sophisticated engineering is key to unlocking their full therapeutic potential.
- E1A Modification
The E1A gene is central to the adenovirus life cycle, playing a pivotal role in viral replication and transforming host cells. By strategically modifying or deleting specific regions within the E1A gene, engineers can achieve tumor selectivity. Many oncolytic adenoviruses are engineered to be replication-competent only in cells where specific tumor suppressor pathways (like the Rb pathway) are dysfunctional, a common characteristic of many cancer cells.
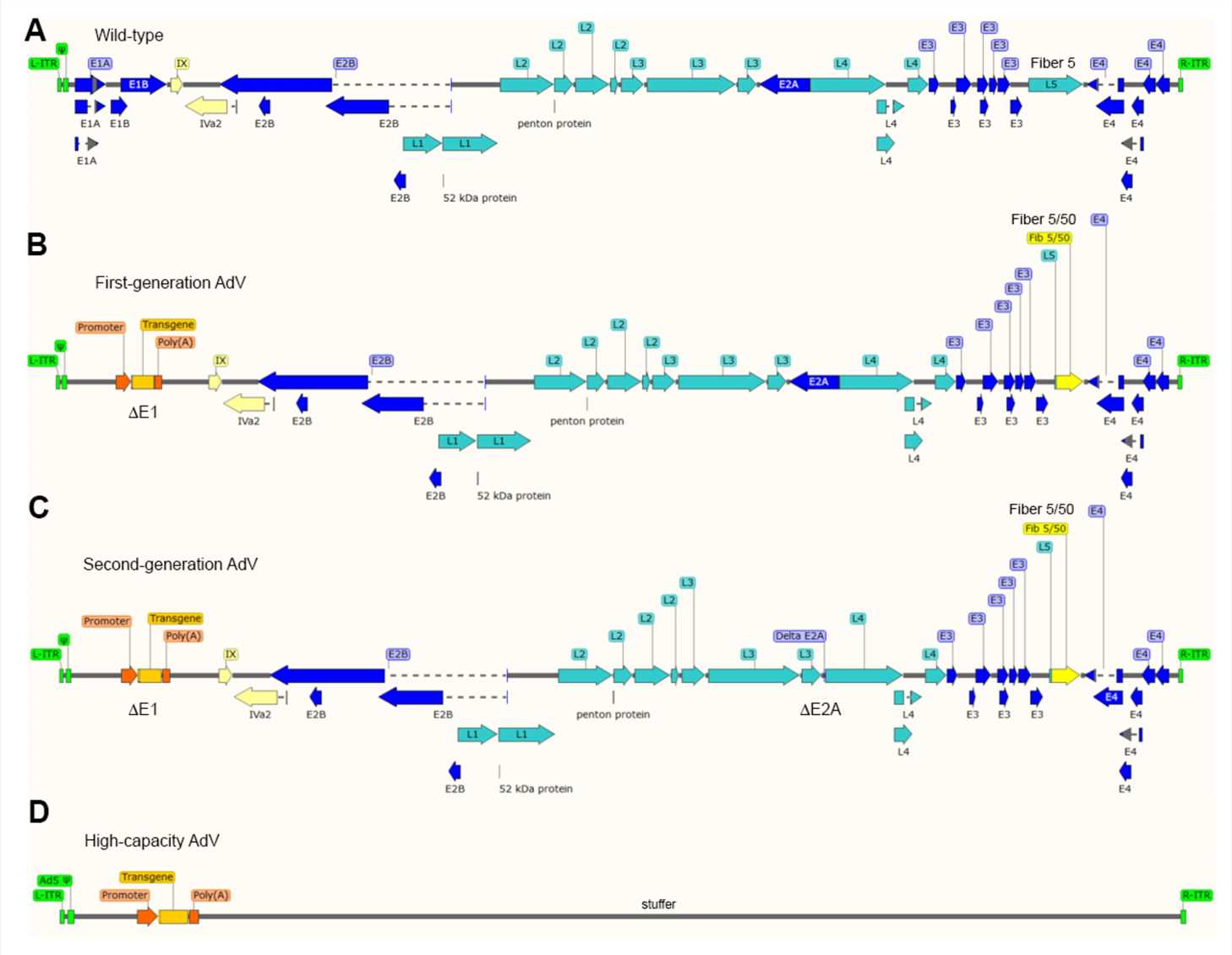 Fig.1 Schematic representation of the genome structures of wild-type (A) and (B) first-generation (E1 deleted), (C) second-generation (E1 and E2A deleted), and (D) third-generation adenovirus vectors.1
Fig.1 Schematic representation of the genome structures of wild-type (A) and (B) first-generation (E1 deleted), (C) second-generation (E1 and E2A deleted), and (D) third-generation adenovirus vectors.1
Beyond E1A, other early adenovirus proteins (E1B, E2, E3, E4) are crucial for various aspects of the viral life cycle, including host immune evasion, viral DNA replication, and regulation of gene expression. Controlling their expression is another sophisticated strategy for enhancing safety and selectivity:
- E1B-55K Modifications
Deleting E1B-55K enables viral replication in p53-defective tumor cells, as functional p53 in healthy cells triggers apoptosis to limit viral spread. This exploits tumor-specific p53 mutations for selective oncolysis.
- Promoter Selectivity
Placing essential genes under tumor-specific promoters restricts viral replication to cancer cells. Active only in the tumor microenvironment, this strategy minimizes off-target activity and strengthens safety profiles.
- Immune Modulation
Engineering the E3 region can suppress host antiviral defenses or express immunostimulatory molecules. These balance reducing vector immunogenicity with enhancing anti-tumor immunity, avoiding excessive neutralization while boosting therapeutic efficacy.
- Gene Editing for Precision Targeting
Gene editing strategies precisely control viral replication to confine effects to tumor cells. Tumor-specific promoters (e.g., telomerase, alpha-fetoprotein) are integrated upstream of viral proliferation-essential genes, restricting their expression to tumor cells and enhancing safety and selectivity.
- Capsid Protein Engineering
Modifying the viral capsid enables targeted delivery and reduces off-target infection. Receptor retargeting via inserting ligands (e.g., RGD peptides for integrins) into the fiber knob domain alters binding specificity, promoting preferential tumor cell infection and enhancing therapeutic precision.
- miRNA Regulation
Leveraging endogenous cellular mechanisms adds control: specific miRNA binding sites are introduced into the viral genome. Highly expressed in healthy tissues, these miRNAs degrade viral transcripts there, while low levels in tumors allow normal replication, enhancing selectivity.
- Synergistic Genetic Arming and Conditional Replication Control
Advanced strategies combine genetic arming and conditional replication for maximal efficacy/safety: adenoviruses carry therapeutic genes (e.g., pro-apoptotic, cytokines) controlled by tumor-specific promoters, releasing factors only in tumors. Combined with gene deletions/promoter control, these vectors selectively replicate and enhance anti-tumor effects while sparing healthy tissue.
Workflow
| Required Starting Materials | Initial Consultation & Project Scoping |
|---|---|
|
Begin with an in-depth discussion to understand your specific therapeutic goals, target indications, and unique requirements. Define the project scope, including a selection of appropriate adenovirus serotypes and initial modification strategies. |
| Vector Design & Advanced Engineering | In Vitro Validation |
| Leverage expertise in molecular biology to design and implement advanced genetic modifications to the adenoviral capsid and genome to enhance tumor selectivity attenuate replication in healthy cells, and integrate desired transgene constructs. | Subject-engineered vectors to rigorous in vitro testing in relevant cancer cell lines and primary healthy cells. Assess replication kinetics, oncolytic potency, transgene expression, and safety and selectivity profiles. |
| Preclinical Evaluation & Immunogenicity Assessment | Data Analysis and Reporting |
| Conduct preclinical studies using relevant animal models to evaluate in vivo efficacy, biodistribution, and systemic safety. Assess the host immune response to the modified vectors to ensure reduced immunogenicity and a prolonged therapeutic window. | Meticulously analyze all experimental data and compile them into detailed reports to provide a clear understanding of the engineered vector's characteristics, performance, and translational potential. Offer insights and recommendations for further development. |
| Final Deliverables | Estimated Timeframe |
|
The typical timeframe for this service ranges from 10-15 weeks, depending on the complexity of the engineering required, the number of constructs to be optimized, and the scope of in vivo studies. |
What We Can Offer
At Creative Biolabs, we understand that each oncology project is unique. That's why our service is not just a standard offering, but a customizable partnership designed to meet your precise therapeutic goals. Leveraging our unparalleled expertise, we offer a comprehensive suite of capabilities to deliver oncolytic adenoviruses with an optimized therapeutic index:
Integrated End-to-End Solutions
From initial concept and advanced design to in vitro validation and preclinical evaluation, we provide a seamless, one-stop service for engineering your oncolytic adenovirus.
Precision Engineering for Unmatched Specificity
Our team employs cutting-edge capsid and genome modification strategies, including sophisticated E1A and early protein control techniques, to ensure your viral vectors exhibit exceptional tumor selectivity and minimal off-target activity.
Robust Immunogenicity Management
We specialize in developing strategies to reduce host immunogenicity, thereby improving vector persistence, enabling repeat dosing, and maximizing the long-term efficacy of your oncolytic therapy.
Optimized Transgene Integration & Expression
With expertise in codon usage optimization and promoter engineering, we ensure efficient and tumor-specific delivery and expression of your therapeutic transgenes, amplifying the anti-tumor effect.
Scalable & GMP-Ready Production Expertise
Our advanced processes ensure efficient production of engineered viral vectors, from laboratory to preclinical scale, adhering to stringent quality standards and preparing for future GMP certification.
Rigorous Quality by Design (QbD) & Analytics
We integrate Quality-by-Design principles and advanced process analytical techniques (PAT) throughout the engineering and validation workflow, guaranteeing superior product quality, stability, and reproducibility.
Comprehensive Characterization & Documentation
You receive meticulously detailed data packages, robust stability assessments, and comprehensive documentation, all designed to support your regulatory submissions and accelerate your journey to the clinic.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
At Creative Biolabs, we are dedicated to advancing the field of oncolytic virotherapy through intelligent design and rigorous engineering. Our focus on Enhancing Safety and Tumor Selectivity ensures that our clients receive best-in-class oncolytic adenovirus constructs with optimized profiles for clinical translation. We pride ourselves on delivering solutions that push the boundaries of cancer treatment, offering therapies that are both highly effective and exceptionally safe. Partner with us to transform the future of oncology.
Click here to reach our inquiry page!
Related Sections
 Distributed under Unsplash License, from Unsplash. Enhancing Tumor Tropism
Distributed under Unsplash License, from Unsplash. Enhancing Tumor Tropism
 Distributed under Unsplash License, from Unsplash. Enhancing Efficacy
Distributed under Unsplash License, from Unsplash. Enhancing Efficacy
Reference
- Tasca, Francesca, Qian Wang, and Manuel AFV Gonçalves. "Adenoviral vectors meet gene editing: a rising partnership for the genomic engineering of human stem cells and their progeny." Cells 9.4 (2020): 953. DOI: 10.3390/cells9040953. Distributed under Open Access license CC BY 4.0, without modification.
