One-Stop Oncolytic Virus Enhancement Services
How Our Service Can Assist the Client
Driven by challenges in oncolytic virus projects such as long development cycles, suboptimal viral spread and efficacy, host immune responses, and complex combination therapy design, our One-Stop Oncolytic Virus Enhancement Service offers integrated solutions. Leveraging advanced viral engineering, targeted extracellular matrix modulation, and nanoengineering platforms, Creative Biolabs accelerates development by optimizing viral pharmacokinetics, enhancing tumor penetration and specificity, and circumventing immune clearance. Tailored to project needs, the service addresses critical barriers in virotherapy, delivering improved therapeutic indices through superior viral spread in solid tumors, optimized systemic delivery profiles, and effective strategies to overcome antiviral immunity, ultimately advancing cancer immunotherapy toward better clinical outcomes.
[Discover How We Can Help - Request a Consultation]
One-Stop Oncolytic Virus Enhancement Service
Optimizing Key Oncolytic Virus Platforms
Our expertise extends across various oncolytic virus platforms, allowing us to enhance the most widely studied and clinically relevant backbones:
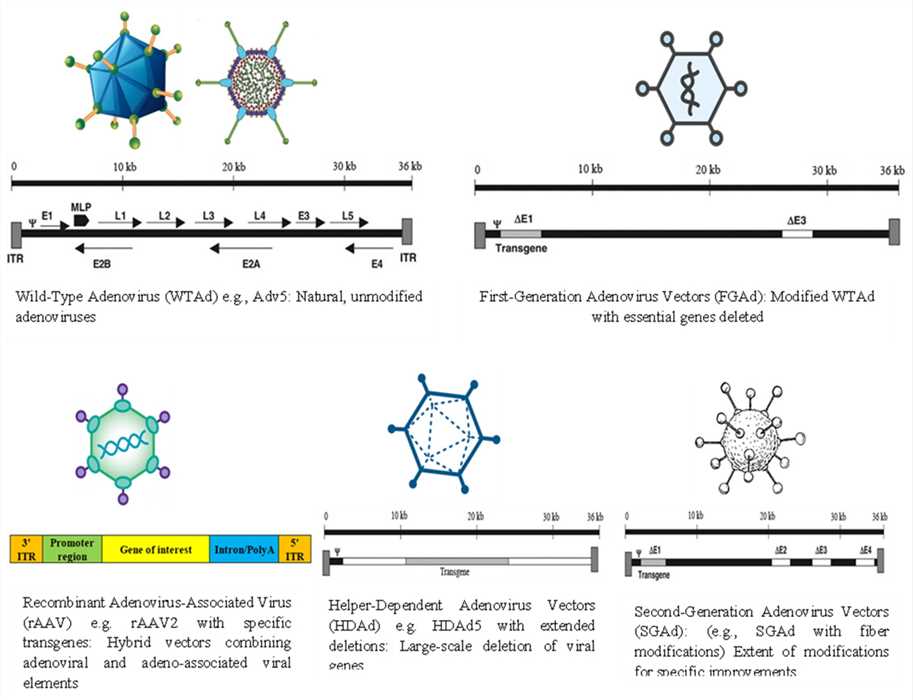 Fig.1 Wild-type adenovirus and different genetically modified adenovirus vectors.1,3
Fig.1 Wild-type adenovirus and different genetically modified adenovirus vectors.1,3
- Modified Adenovirus: We genetically engineer adenoviruses for enhanced tumor selectivity and payload capacity: deleting viral genes (e.g., E1B, E3) to restrict replication to cancer cells, inserting therapeutic genes/promoters for controlled replication and efficacy, and modifying capsid proteins to improve tumor targeting and reduce immunogenicity.
- Modified Vaccinia Virus: We engineer vaccinia virus variants with enhanced tumor-specific replication, systemic delivery, and immunomodulation by leveraging its large genomic capacity and high replication rates, incorporating multiple therapeutic genes, reducing clearance, and expressing molecules to reprogram the tumor microenvironment.
- Modified Herpes Simplex Virus (HSV): We engineer HSV-1 vectors by deleting normal-cell replication-essential genes (e.g., γ34.5, ICP6) to retain tumor-specific replication. Services include incorporating genes for prodrug-converting enzymes, immune-stimulatory cytokines, or matrix-degrading enzymes (e.g., Chase-ABC) to enhance oncolytic potency and solid tumor spread.
Creative Biolabs' One-Stop Oncolytic Virus Enhancement Service provides a multifaceted approach to elevate the therapeutic potential of oncolytic viruses, addressing key challenges in cancer therapy.
Emerging Strategies for Enhancing OV Efficiency
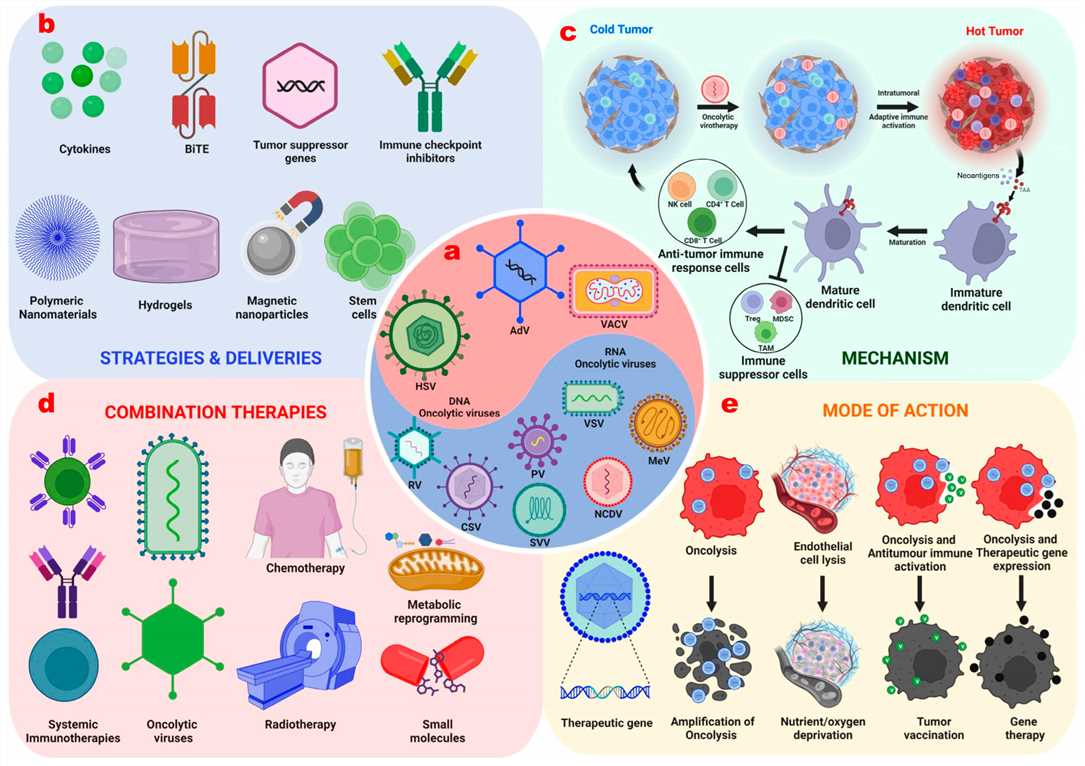 Fig.2 Strategies to enhance the therapeutic potential of oncolytic viruses.2,3
Fig.2 Strategies to enhance the therapeutic potential of oncolytic viruses.2,3
- Increasing Viral Transduction and Replication Rates
We employ advanced genetic engineering to optimize viral entry, replication, and progeny release within tumor cells. This involves incorporating elements that enhance viral protein synthesis and assembly, leading to a higher burst size and accelerated infection cycles. This targeted approach ensures that the virus proliferates efficiently within the tumor, maximizing its cytotoxic effect.
-
Enhancing Virus Selectivity to Tumor Cells
- Transcriptional Targeting: Utilizing tumor-specific promoters to drive viral gene expression only in malignant cells.
- Receptor Retargeting: Engineering viral entry mechanisms to bind to receptors uniquely or highly expressed on cancer cell surfaces.
- MicroRNA Detargeting: Incorporating microRNA binding sites that are abundant in healthy cells but absent in tumor cells, leading to selective attenuation of viral replication in normal tissues. These methods ensure that the virus primarily infects and lyses cancerous cells, enhancing safety and therapeutic index.
-
Combined Therapy with Other Therapeutic Agents and Modalities
- Arming OVs with Therapeutic Payloads: Genetically engineer OVs to express immunomodulatory molecules (e.g., cytokines, immune checkpoint inhibitors), pro-apoptotic genes, or prodrug-activating enzymes, transforming tumor cells into in situ bioreactors for local therapeutic delivery.
- Integration with Radiotherapy and Chemotherapy: Design OVs to enhance chemo/radiotherapy via immunogenic cell death induction, tumor cell sensitization to genotoxic stress, or improved drug penetration. Use nanoengineering (e.g., co-encapsulating OVs and chemotherapeutics in nanocarriers) for targeted co-delivery and synergistic effects.
-
Suppressing Cellular Antiviral Immunity
- Engineering Immune Evasion Strategies: Modifying OVs to express molecules that counteract the host's innate antiviral responses, such as inhibiting type I interferon signaling pathways.
- Nanoparticle Encapsulation: Utilizing nanocarriers to shield the oncolytic virus from neutralizing antibodies and complement system components in circulation, extending its half-life and improving systemic delivery to the tumor.
What we can offer
At Creative Biolabs, we offer a comprehensive suite of capabilities tailored to accelerate your oncolytic virus programs. Our One-Stop Oncolytic Virus Enhancement Service combines cutting-edge science with a client-centric approach, ensuring customized solutions for your unique challenges.
- Customized Viral Engineering: Design bespoke genetic modifications for OVs to optimize entry, replication, and release, tailored to tumor targets and viral backbones.
- Targeted Extracellular Matrix Modulation: Incorporate ECM-degrading enzymes into OVs to enhance spread and distribution in solid tumors.
- Optimized Viral Pharmacokinetics: Modulate host antiviral pathways to improve systemic delivery, tumor accumulation, and viral persistence.
- Advanced Nanoengineering Integration: Use liposomal/hydrogel encapsulation to shield OVs from immune clearance and enable tumor-targeted delivery.
- Enhanced Tumor Selectivity: Employ transcriptional targeting, receptor retargeting, and microRNA retargeting to increase cancer-cell specificity and reduce off-target effects.
- Synergistic Combination Therapy Development: Integrate OVs with chemotherapy, radiotherapy, or immune checkpoint inhibitors for multi-modal cancer treatments.
- Rigorous Preclinical Validation: Conduct comprehensive in vitro/in vivo testing for efficacy, PK, and safety data, supported by a robust quality system.
Advantages
Choosing Creative Biolabs for your oncolytic virus enhancement means partnering with a team that combines over 20 years of biology specialization with cutting-edge scientific innovation. Our unique advantages ensure your project's success:
- Integrated "One-Stop" Solution: Cover all stages from design to preclinical validation, simplifying vendor coordination.
- Proven Efficacy Enhancement: ECM degradation boosts viral titer 10-fold; pathway modulation increases RNA 150-fold in preclinical models.
- Focus on Clinical Translatability: Prioritize safety, specificity, and therapeutic index for seamless bench-to-bedside translation.
- Safety-First Approach: Rigorous testing ensures enhanced efficacy without compromising oncolytic virus safety in normal tissues.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
Workflow
| Required Starting Materials | Project Scoping & Design |
|---|---|
|
We begin with an in-depth consultation to understand your project goals, specific requirements, and any existing constraints. Based on this, we design a customized oncolytic virotherapy development strategy, including virus selection, modification, and combination therapy protocols. |
| Viral Engineering & Construction | In Vitro Validation |
| This step involves precise genetic modification of oncolytic viruses, inserting genes, and modulating pathways. Advanced nano-arming, like liposomal/hydrogel encapsulation, enhances delivery and targeting. | Through conducting comprehensive in vitro assays on enhanced viruses, evaluating cytotoxicity, replication kinetics, spread in 2D/3D models, and initial immune interactions like IFN response and T-cell activation. |
| In Vivo Preclinical Evaluation | Data Analysis and Reporting |
| Engineered viruses are tested in animal models for in vivo performance, covering pharmacokinetic profiling, antitumor efficacy, safety assessments, and TME analysis via flow cytometry and gene expression. | In the data analysis and reporting phase, our team offers comprehensive data interpretation with statistical analysis and scientific insights. You'll receive detailed experimental reports including raw/processed data, graphical presentations, conclusions, and optimization/clinical translation suggestions. |
| Final Deliverables | Estimated Timeframe |
|
Detailed preliminary experimental report: experimental procedures, results analysis documents; Oncolytic virus product: oncolytic virus preparation after completion of the boost; |
A comprehensive service project typically takes 12-24 weeks, varying by viral engineering complexity and preclinical evaluation scope, including constructs, in vivo models, and immunological analysis. |
[Contact Our Team for More Information and to Discuss Your Project]
Creative Biolabs' One-Stop Oncolytic Virus Enhancement Service provides an unparalleled, integrated solution for overcoming the critical challenges in oncolytic virotherapy. From innovative viral engineering and targeted delivery mechanisms to comprehensive preclinical validation, we empower our clients to develop safer, more effective, and clinically translatable oncolytic virus therapies that revolutionize cancer treatment.
References
- Salauddin, Md, et al. "Clinical Application of Adenovirus (AdV): A Comprehensive Review." Viruses 16.7 (2024): 1094. DOI: 10.3390/v16071094
- Muthukutty, Palaniyandi, and So Young Yoo. "Oncolytic virus engineering and utilizations: cancer immunotherapy perspective." Viruses 15.8 (2023): 1645. DOI: 10.3390/v15081645
- Distributed under Open Access license CC BY 4.0, without modification.
