Introduction How Can We Help? Deliverables Key Benefits Related Products FAQs Contact
Are you currently facing long drug development cycles, challenges in achieving site-specific drug delivery, or issues with uncontrolled systemic toxicity in your payload delivery? Creative Biolabs' ApDC Development service helps you accelerate drug discovery, significantly reduce off-target toxicity, and obtain high-purity, structurally precise conjugates through our Automated Modular Solid-Phase Synthesis and Advanced Half-Life Extension platforms. This chemical approach bypasses the limitations of traditional antibody-based systems, offering unparalleled therapeutic control.
Overview of ApDC Development
Nucleic acid aptamers are single-stranded oligonucleotides that function as "chemical antibodies," selected to bind with high specificity and affinity to a target molecule or cell surface marker. These features position them as ideal targeting ligands for precision medicine due to their small size, which facilitates superior tissue and tumor penetration.
What Is Our Service?
Creative Biolabs' Aptamer-Small Molecule Drug Conjugate (ApDC) Development service specializes in the chemical engineering and synthesis of these conjugates. We leverage automated chemistry to covalently link a high-affinity aptamer to a cytotoxic or therapeutic small molecule via a specialized, often cleavable, linker, ensuring a product with verifiable purity and consistency. Core Applications of Our Services:
-
Targeted Oncology: Delivering potent cytotoxic small molecules specifically to cancer cells overexpressing target receptors, thereby minimizing systemic exposure and side effects.
-
Intracellular Delivery: Engineering aptamers for active internalization into target cells, enabling the precise, targeted delivery of drugs and other genetic payloads.
-
Advanced Diagnostics: Creating stable, easily modified probes that offer superior thermal stability and tissue penetration for highly specific in vivo imaging and biomarker detection.
Why Choose Us?
Our ApDC platform offers a definitive, chemically precise alternative.
|
Pain Points
|
Benefit Created by Creative Biolabs
|
|
Inconsistent Drug Loading
|
We guarantee an exact, defined Drug-to-Carrier Ratio for every molecule, minimizing batch-to-batch variability and ensuring a homogeneous drug product.
|
|
Manufacturing Instability & Cost
|
Aptamers are synthesized chemically, leading to lower cost, guaranteed high purity, and superior thermal/chemical stability compared to biologics.
|
|
Poor Tumor Penetration
|
The smaller size ensures rapid, efficient penetration into dense solid tumor tissues, which are often inaccessible to large antibodies.
|
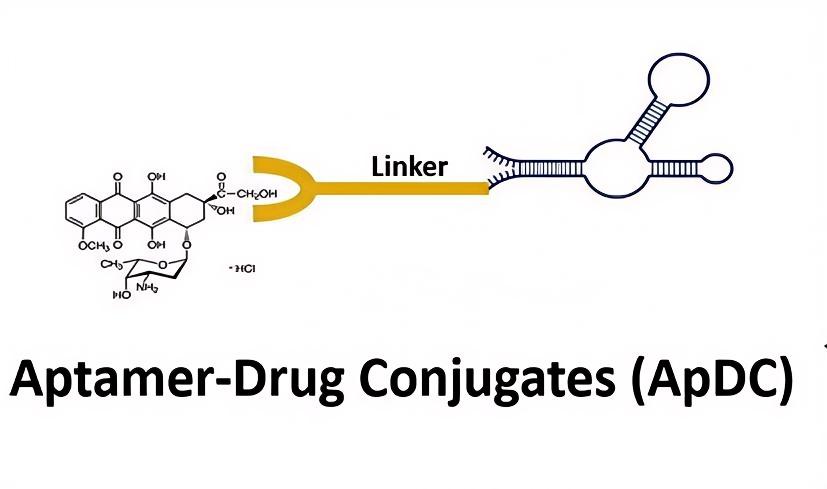 Fig.1 Structure of ApDC.1
Fig.1 Structure of ApDC.1
How Creative Biolabs' ApDC Development Can Assist Your Project?
Our service provides a comprehensive, end-to-end pathway for developing functionally optimized, clinically viable ApDC candidates.
Workflow
-
Required Starting Materials
-
Target Biomarker/Protein
-
Small Molecule Payload
-
Desired cell line
-
Target & Ligand Selection
-
Therapeutic Module Synthesis
-
Automated Solid-Phase Conjugation
-
Stabilization & Half-Life Extension
-
Quality Control & Functional Validation
Discover Your Project's Capabilities – Contact Us for Pricing and Availability Now!
Deliverables
Upon completion of your project, Creative Biolabs provides a complete, transparent package designed to seamlessly advance your candidate into preclinical studies:
-
Final Purified ApDC Product: The specified quantity of the chemically defined, high-purity Aptamer-Small Molecule Drug Conjugate.
-
Comprehensive Synthesis Report.
-
Quality Control (QC) and Analysis Report: Full analytical package, including purity analysis and Endotoxin testing.
-
In Vitro Functional Data: Summary report on target-specific binding affinity and targeted cytotoxicity.
Key Benefits
Creative Biolabs' ApDC service leverages chemical innovation to overcome the structural, manufacturing, and stability hurdles inherent in traditional biologics, providing a superior platform for targeted therapy development.
High Scalability and Purity
Our automated solid-phase synthesis is highly efficient and scalable, providing high purity and ensuring consistency from research scale to industrial production.
Enhanced Stability
Aptamers offer inherent high thermal and chemical stability. We further enhance this through strategically placed 2'-modifications.
Advanced Linker Systems
We offer customizable linker chemistries, providing spatiotemporal control over drug release at the target site, minimizing off-target activity.
Fast-Tracked Timelines
Leveraging the speed of chemical synthesis over biological production, we dramatically reduce the time required to generate lead candidates.
Reveal Your Project's Possibilities – Request Pricing and Availability Today!
Related Products
Frequently Asked Questions
Q1: What is the main advantage of an ApDC over a classic ADC for a small molecule payload?
A: The main advantage is precision and control. Unlike ADCs, where drug conjugation is often heterogeneous, our ApDCs are synthesized using an automated, modular chemical process that allows us to define the exact location and number of drug molecules with high fidelity. Furthermore, ApDCs offer superior tumor penetration due to their small size and lack the immunogenicity risk associated with large protein therapeutics.
Q2: Can you develop an ApDC for a target where no suitable antibody exists?
A: Yes, absolutely. Aptamers are screened via SELEX, which does not depend on the biological host's immune response. This allows us to generate high-affinity aptamers against challenging targets, including small molecules or low-immunogenic surface markers, opening up therapeutic possibilities for previously "undruggable" biomarkers.
Q3: Are there any concerns regarding the potential toxicity of the aptamer component itself?
A: Aptamers, being short nucleic acid sequences, have demonstrated extremely low immunogenicity and systemic toxicity compared to traditional antibody scaffolds. Furthermore, their rapid clearance (when unmodified) acts as an inherent safety feature. Our stabilization modifications are carefully selected to maintain this non-immunogenic profile, ensuring a better overall safety margin.
Contact Us
Creative Biolabs is committed to driving the future of precision medicine by offering chemically superior ApDC development solutions. Our platform ensures that your therapeutic payload is delivered with unparalleled specificity, stability, and manufacturing consistency, accelerating your journey from concept to clinic. To learn more about our proprietary ApDC technology and discuss your specific drug candidate needs, please reach out to our team of bioconjugation specialists.
Reference
-
Park, Dongsik, Su Jin Lee, and Jee-Woong Park. "Aptamer-based smart targeting and spatial trigger–response drug-delivery systems for anticancer therapy." Biomedicines 12.1 (2024): 187. Distributed under an Open Access license CC BY 4.0, the original image was cropped. DOI: https://doi.org/10.3390/biomedicines12010187.
For Research Use Only.
Related Sections:

 Fig.1 Structure of ApDC.1
Fig.1 Structure of ApDC.1