Why C3b and iC3b Deposition Matters
C3 is the hub of the complement cascade. Once activated, it converts to C3b, a potent opsonin that tags targets for clearance, amplifies the cascade, and drives downstream events (C5 convertase formation and, ultimately, MAC assembly). Regulated cleavage of C3b yields iC3b, C3c, and C3dg, each carrying distinct biological consequences for phagocytosis, immune cell signaling, and immunogenicity. Measuring where, when, and how strongly C3 fragments deposit answers pivotal questions.
-
Does a therapeutic antibody trigger classical-pathway opsonization on target cells?
-
Will a lipid nanoparticle, AAV capsid, exosome, or polymeric scaffold be rapidly opsonized in blood?
-
Are new small-molecule or biologic candidates effectively modulating complement?
-
How do pathway-specific conditions (classical, lectin, alternative) shape a product's complement footprint?
Great Minds Choose Creative Biolabs
Add-On Assays That Sharpen Interpretation
Design Your Customization
Case Studies
Case 1
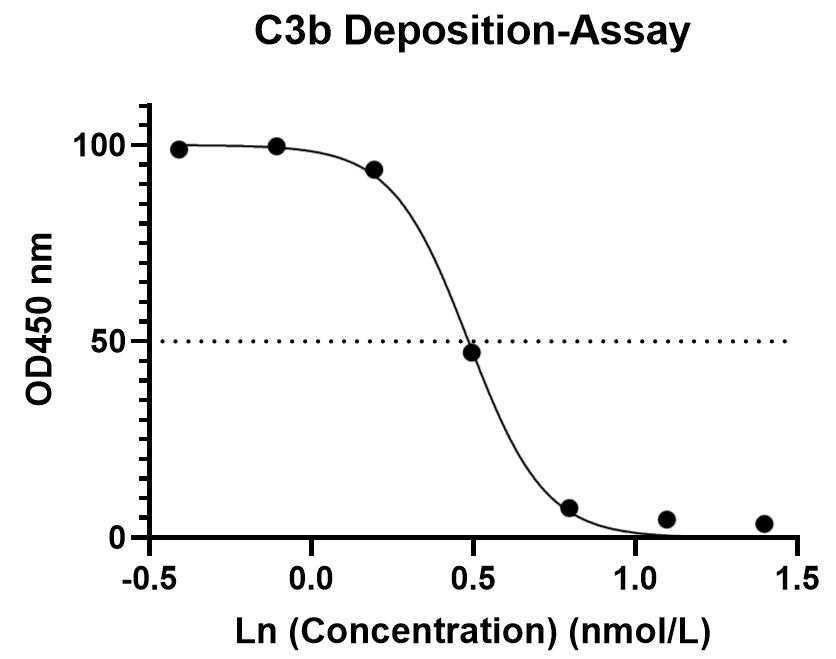
C3b deposition assay test in the normal human serum
Creative Biolabs conducted C3b deposition assays on client-provided samples. The following figure illustrates a representative C3b deposition assay. The results indicated that the sample inhibited C3b deposition in normal human serum. This assay was performed using seven serial dilutions, with each dilution tested in duplicate. Further customization of the experimental plan can be accommodated based on specific requirements.
Fig. 2 C3b deposition assay test in the normal human serum.
Case 2
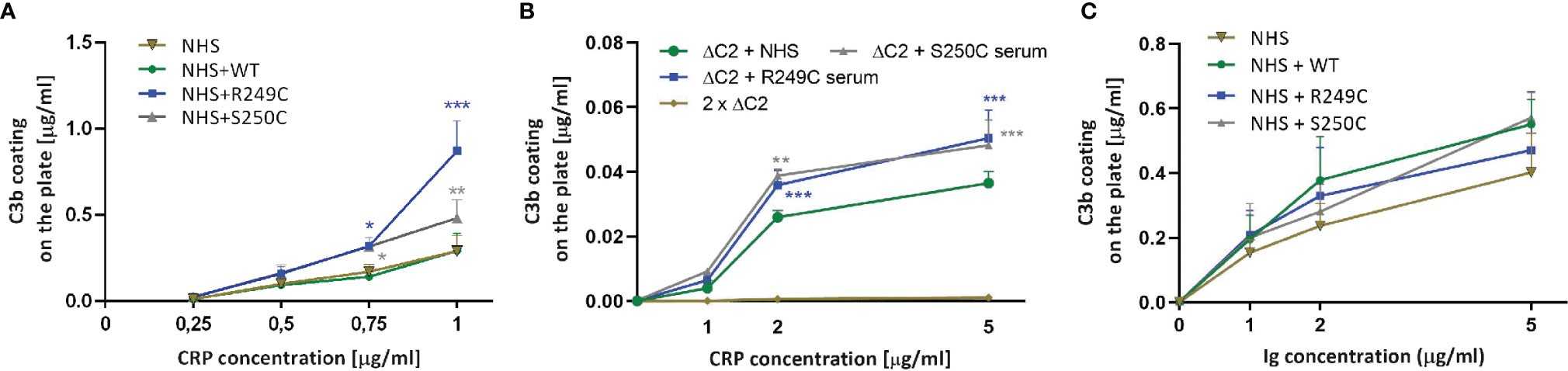
C3b deposition assay in the presence of CRP and human Ig preparation
Increasing concentrations of CRP were coated onto the ELISA microplate and overlaid with normal human serum +/− recombinant C2 variants. C3b deposition was detected by anti-C3b antibody. Purified C3b directly coated on the plate was used as a standard.
Fig. 3 C3b deposition assay in the presence of CRP and human Ig preparation.
1,2
References
-
Urban, Aleksandra, et al. "Gain-of-function mutations R249C and S250C in complement C2 protein increase C3 deposition in the presence of C-reactive protein." Frontiers in Immunology 12 (2021): 724361. https://doi.org/10.3389/fimmu.2021.724361
-
Distributed under Open Access license CC BY 4.0, without modification.
Frequently Asked Questions
How do you decide between plate-based C3b deposition and flow cytometry opsonization?
Plate formats are ideal for high-throughput ranking of purified antigens, immune complexes, and coated materials with tight CVs and straightforward normalization. Flow cytometry is best when biological context matters—intact cells, microbes, EVs, LNPs, or AAV—because you gain single-event resolution, subpopulation gating, and easier translation to ADCP/ADNP. Many programs start on plates for speed, then confirm on flow for biological relevance.
What sample types and species can you work with?
Serum is standard. We also support NHP, mouse, and rat with validated cross-reactive detection. For plasma, citrate can be used with caveats; EDTA plasma is unsuitable for activation readouts. We routinely test purified antibodies/proteins, intact cells, bacteria/yeast, EVs, LNPs, AAV, coated coupons, and microfluidic surfaces.
Can you correlate C3b deposition with CH50/AH50, CDC, or phagocytosis?
Absolutely. Many clients request a cascade-spanning panel: C1q (priming)-C3b/iC3b (opsonization)-sC5b-9 (terminal) plus a functional endpoint (CH50/AH50, CDC, or ADCP/ADNP).
Can you distinguish C3b from iC3b and C3d, and why does that matter?
Yes. We deploy fragment-specific antibodies and orthogonal checks to separate C3b, iC3b, and C3d. Mapping this trajectory clarifies convertase activity, Factor I-mediated regulation, and persistence of opsonization signals that can influence downstream immune readouts and biomaterial compatibility.
What do you deliver at closeout?
You will receive an interpretive report (methods, figures, conclusions), raw files, tidy processed tables, and a QC dossier (acceptance criteria performance, deviations, corrective actions, reagent lots). Others are available on request.
What is the typical timeline from kickoff to report?
The timelines are contingent on sample availability, species, and scope. If you're milestone-driven, we propose phased gates to de-risk timelines.
Do you support downstream functional assays if C3b deposition is positive?
Definitely. We commonly add CDC or CH50/AH50 to link opsonization to effect. We can include sC5b-9 to assess terminal pathway progression and recommend design tweaks that reduce unwanted complement activation.














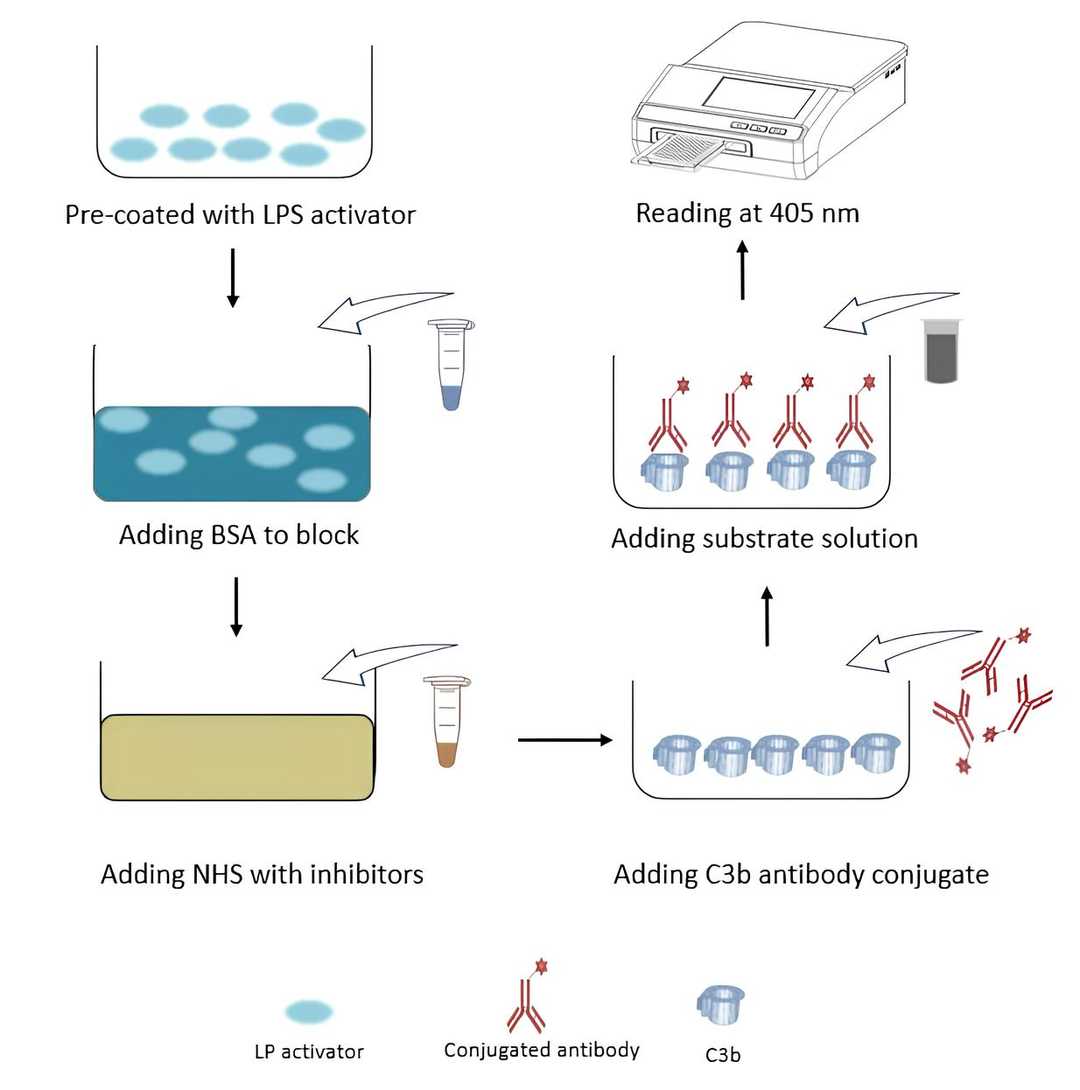 Fig. 1 Workflow of ELISA-based C3b deposition assay.
Fig. 1 Workflow of ELISA-based C3b deposition assay.