Introduction to ASOs
Antisense oligonucleotides (ASOs) modulate gene expression by mechanisms including RNA degradation by RNase H, translation inhibition, or modification of splicing. Their high specificity has made them indispensable in research whereby they are employed for the knockdown of genes and study of specific RNA functions both in vitro and in vivo.
Looking forward, the combination of ASOs with gene-editing technologies for gene therapy such as CRISPR has great potential to allow for even more precise gene regulation.
Key Mechanisms & Applications
RNase H Degradation
ASOs bind to target RNA, triggering RNase H-mediated degradation and reducing protein expression.
Translation Inhibition
Acting as steric blockers to physically prevent the ribosome from translating the mRNA.
Splicing Modification
Modifying pre-mRNA splicing to include or exclude exons, useful in correcting genetic diseases.
Custom Antisense Oligonucleotide Synthesis Solution
Strategy & Design
Comprehensive Design Strategy
Our team works closely with clients to understand their research or therapeutic goals. We assist in the design of antisense oligonucleotides, considering factors such as target RNA sequence, chemical modifications, and potential off-target effects.
Bioinformatics tools are used to optimize the oligonucleotide sequence for enhanced stability, binding affinity, and reduced immunogenicity. Special attention is given to avoiding secondary structures and unintended interactions.
Synthesis & Modifications
Advanced Chemical Engineering
We offer a variety of chemical modifications to improve ASO stability and efficacy. Options include backbone modifications (phosphorothioates, 2’-O-methyl), terminal modifications, and conjugation for targeted delivery (e.g., GalNAc, peptides).
Using cutting-edge synthesis platforms, the ASO is chemically synthesized and undergoes rigorous purification (e.g., HPLC, ion-exchange chromatography) to ensure high purity and yield tailored to your scale.
QC & Delivery
Rigorous Quality & Support
Every oligonucleotide undergoes stringent quality control testing, including mass spectrometry and sequence verification, to ensure it meets the desired specifications and standards required for downstream applications.
Finalized ASOs are delivered to the client with detailed QC reports. Post-delivery support includes consultation on ASO handling, storage, and experimental setup if required to ensure your success.
Available Chemical Modifications
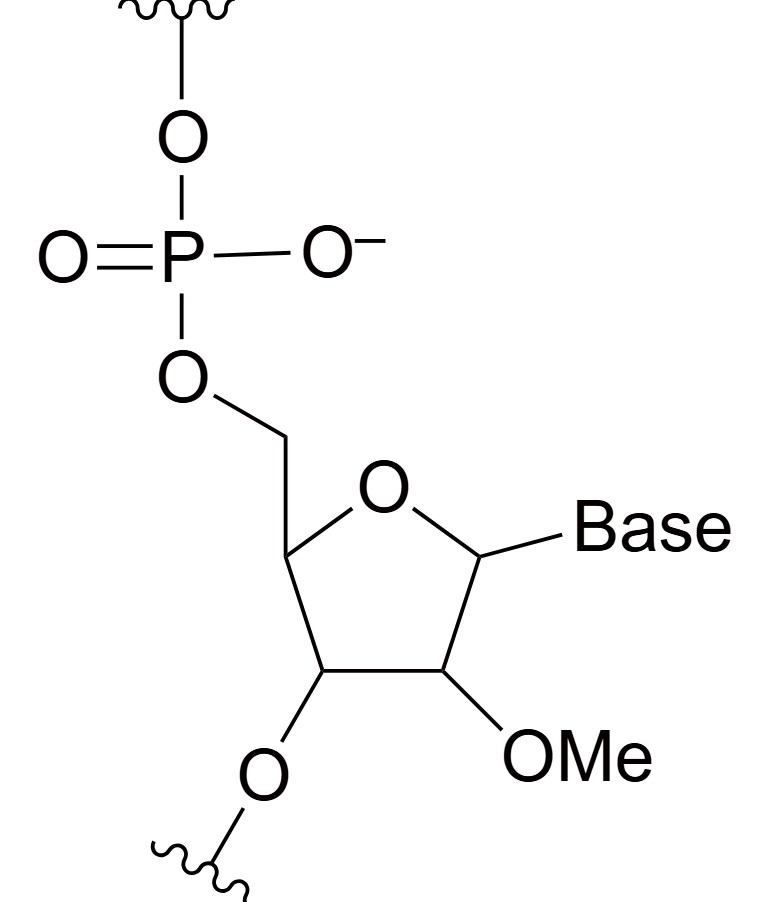
2'-O-Methyl (2'-OMe)
2'-O-Methyl (2'-OMe) RNA bases are chemically modified nucleotides where a methyl group (-CH3) is added to the 2' hydroxyl group of the ribose sugar. This structural alteration serves two primary purposes: increasing the chemical stability of the oligonucleotide and reducing its immunogenicity when introduced into biological systems. By adding a bulky, hydrophobic methyl group, the oligonucleotide becomes more resistant to enzymatic degradation by ribonucleases, which would otherwise cleave unmodified RNA molecules. Furthermore, the 2'-OMe modification decreases the immune recognition of the oligonucleotide, which is especially important in therapeutic applications to avoid immune system activation. This modification is commonly found in antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and other RNA therapeutics, where it enhances the molecule's binding affinity to complementary RNA strands, improves pharmacokinetic properties, and prolongs the therapeutic effect. Its reduced immunogenicity also makes 2'-OMe RNA bases a preferred choice in treatments where minimizing immune response is critical for patient safety.
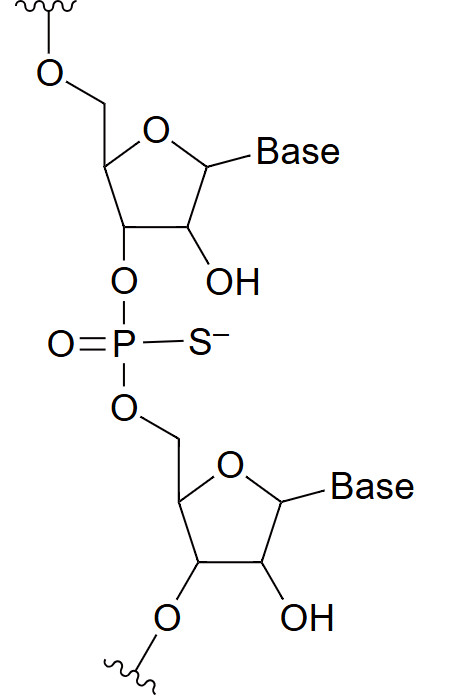
Phosphorothioate (PS)
Phosphorothioate is a backbone modification where a non-bridging oxygen atom in the phosphate group is replaced with a sulfur atom. This modification confers high resistance to nucleases, making them ideal for therapeutic applications requiring sustained in vivo activity. Additionally, the sulfur substitution alters the oligonucleotide's overall charge and conformation, which can improve its interaction with proteins and other cellular components. However, the modification may also slightly reduce the binding affinity of the oligonucleotide for its target RNA or DNA sequence, so careful design is required to balance stability and efficacy in therapeutic applications. Despite this, phosphorothioate modifications remain a cornerstone in oligonucleotide therapeutics due to their excellent durability in physiological conditions.
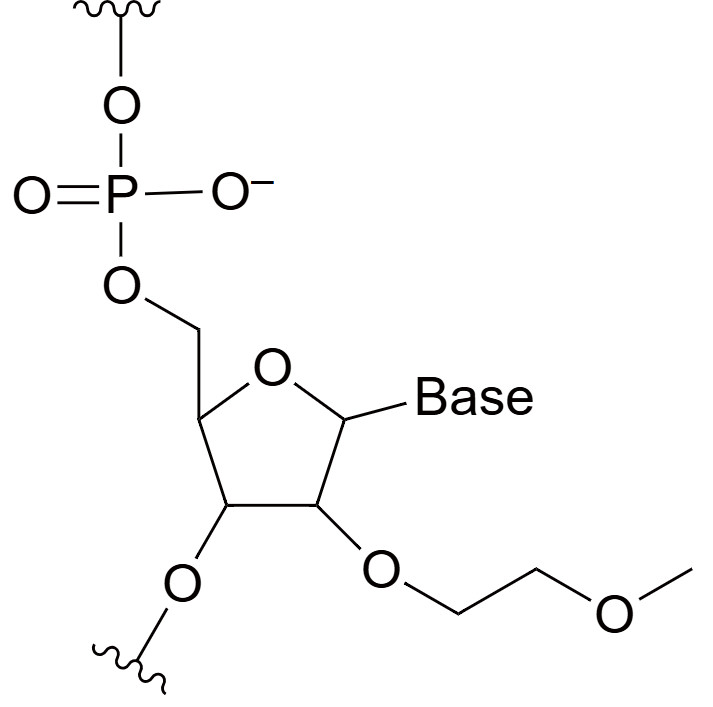
2'-O-Methoxyethyl (MOE)
MOE modifications involve adding a methoxyethyl group to the 2' position of the ribose sugar. This bulky group significantly enhances stability, nuclease resistance, and binding affinity. MOE-modified oligonucleotides have prolonged half-lives in vivo, allowing for less frequent dosing, and are particularly valuable for treating chronic diseases. Additionally, the MOE modification improves the oligonucleotide's pharmacokinetics by enhancing its binding specificity and reducing the likelihood of off-target effects. This modification is particularly valuable in treating chronic diseases, where long-term stability and efficacy are required for sustained therapeutic outcomes.
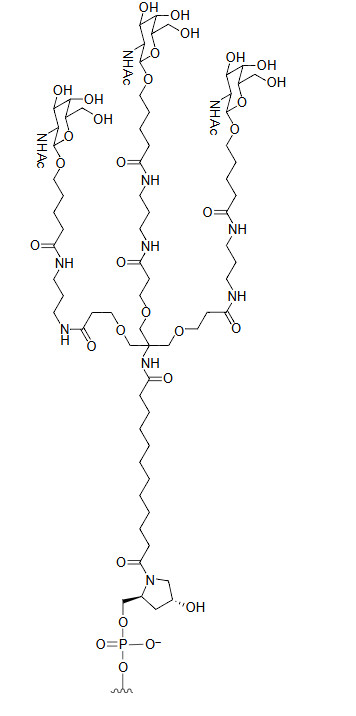
N-acetyl-galactosamine (GalNAc)
GalNAc is a carbohydrate modification for targeted drug delivery to the liver. It allows oligonucleotides to bind with high affinity to the asialoglycoprotein receptor (ASGPR) on hepatocytes. This receptor-mediated delivery ensures precise targeting, improved efficacy, and reduced systemic toxicity, revolutionizing RNA therapeutics for liver-associated diseases. This targeted approach not only improves therapeutic efficacy but also reduces off-target effects and minimizes systemic toxicity. GalNAc conjugates are particularly valuable in the treatment of liver-associated diseases such as hypercholesterolemia, hepatitis, and liver fibrosis, where precise delivery to hepatocytes is essential for successful treatment outcomes.
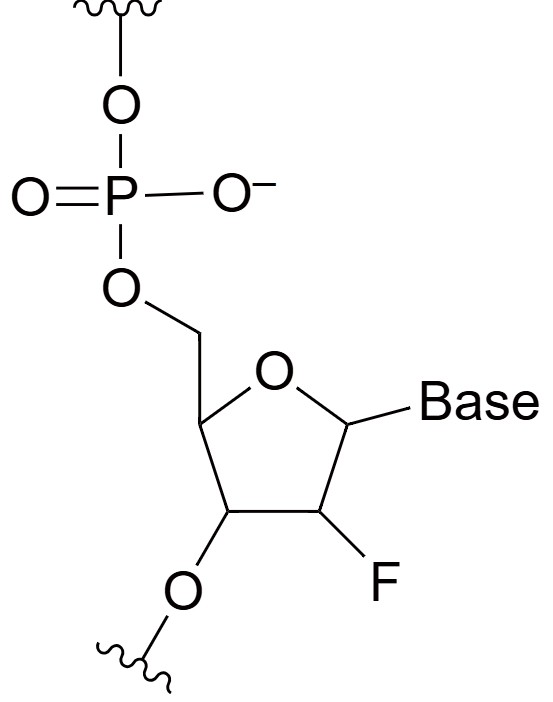
2'-Fluoro (2'-F)
2'-Fluoro (2'-F) modifications involve substituting the hydroxyl group at the 2' position of the ribose sugar with a fluorine atom in adenosine, cytidine, guanosine, or uridine nucleotides. This substitution significantly enhances the stability of the oligonucleotide by increasing resistance to ribonucleases, which typically degrade unmodified RNA molecules. Fluorine is a small, highly electronegative atom that provides chemical stability without significantly altering the base pairing properties of the nucleotide, allowing the modified oligonucleotide to maintain high binding affinity to its target sequence. The increased stability of 2'-F-modified oligonucleotides makes them particularly useful in therapeutic applications, such as siRNAs, antisense oligonucleotides, and aptamers, where long-lasting activity is required. Additionally, 2'-F-modified oligonucleotides exhibit improved pharmacokinetic profiles, including enhanced serum stability and extended half-life, making them more effective in therapeutic settings. These modifications also reduce the immunogenicity of the oligonucleotide, minimizing the risk of immune activation and adverse reactions in patients.
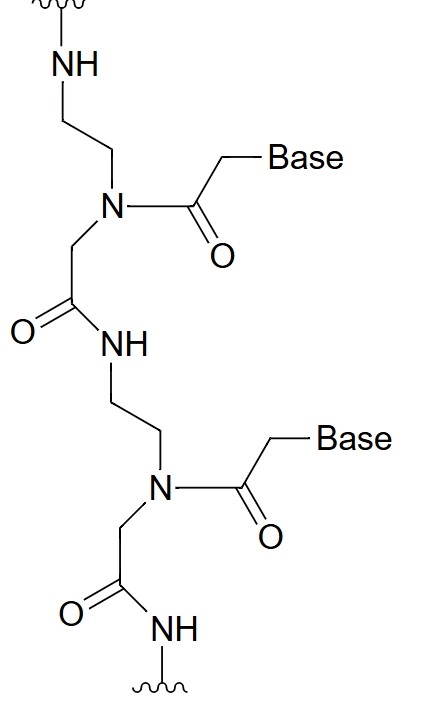
Peptide Nucleic Acid (PNA)
Peptide Nucleic Acid (PNA) is a synthetic nucleic acid analog in which the standard sugar-phosphate backbone of DNA or RNA is replaced by a peptide-like backbone composed of N-(2-aminoethyl) glycine units. This unique structure makes PNAs neutral in charge, allowing for stronger hybridization with complementary DNA or RNA sequences due to the lack of electrostatic repulsion. PNAs exhibit high thermal stability and specificity in binding to complementary strands, making them excellent tools for molecular diagnostics, antisense therapies, and gene editing applications. PNAs are also highly resistant to enzymatic degradation, increasing their durability in biological systems. Due to their synthetic backbone, PNAs are less likely to trigger immune responses, making them suitable for therapeutic uses. PNA oligomers can bind to single-stranded DNA or RNA with high affinity, forming stable duplexes that can interfere with gene expression or inhibit protein synthesis, offering promising applications in gene regulation and targeted therapeutics.
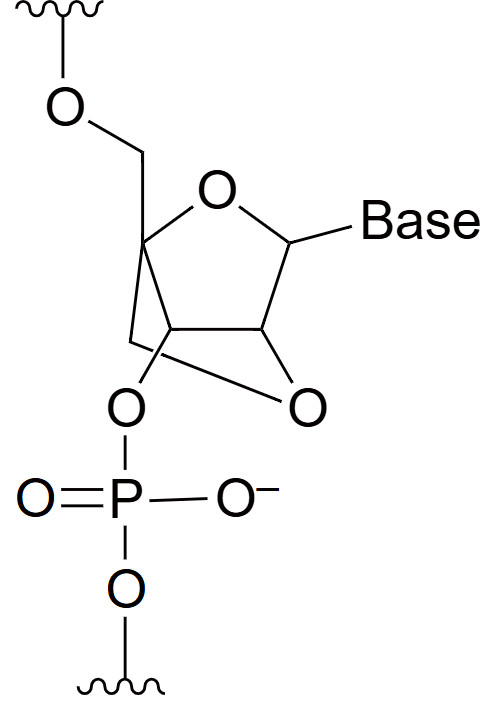
Bridged Nucleic Acid (BNA)
Bridged Nucleic Acid (BNA) is a type of synthetic nucleotide analog in which the ribose sugar is chemically "bridged" between the 2' oxygen and the 4' carbon, locking the sugar in a constrained conformation. This structural modification enhances the thermal stability of oligonucleotides by increasing their binding affinity to complementary nucleic acid strands. BNA modifications, improve the hybridization properties of oligonucleotides, making them more effective at targeting specific DNA or RNA sequences. BNAs are commonly used in antisense oligonucleotides, siRNAs, and molecular probes, where precise binding and resistance to nuclease degradation are critical. The bridged structure reduces the conformational flexibility of the nucleotides, resulting in tighter binding to complementary strands and greater resistance to degradation by enzymes. These properties make BNAs particularly useful in therapeutic applications where stability and specificity are paramount, such as in the development of gene silencing therapies and molecular diagnostics.
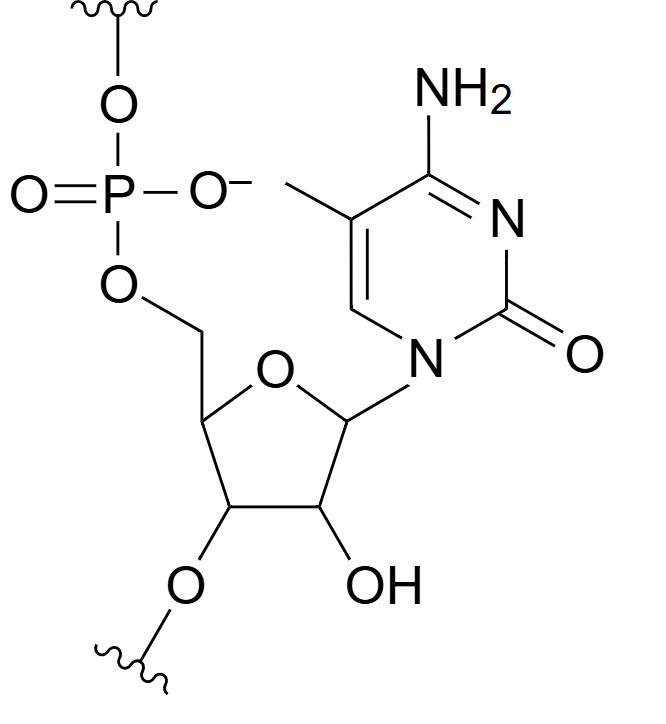
5-Methylcytidine
5-Methylcytidine is a modified cytosine nucleotide in which a methyl group is attached to the 5-carbon position of the cytosine ring. This modification plays a crucial role in epigenetic regulation, as it mimics the natural methylation of cytosine in DNA, which is commonly involved in gene silencing. The methylation of cytidine can influence gene expression by affecting how DNA interacts with transcriptional machinery or by altering chromatin structure. In synthetic oligonucleotides, 5-Methylcytidine enhances hybridization specificity and stability when forming duplexes with complementary strands. This modification increases the thermal stability of the nucleic acid duplex, making it more resistant to denaturation and degradation by nucleases. 5-Methylcytidine is often incorporated into antisense oligonucleotides, siRNAs, and other nucleic acid-based therapeutics to improve binding affinity to target sequences, increase biological stability, and better mimic the natural methylation patterns found in gene regulation studies.
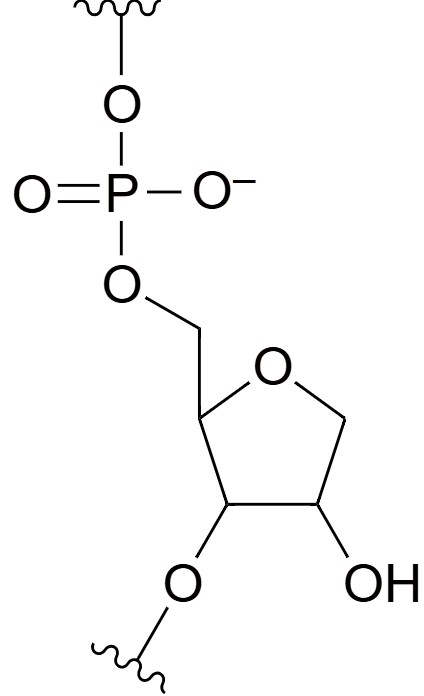
Abasic RNA
Abasic RNA refers to RNA molecules in which a base (adenine, cytosine, guanine, or uracil) has been removed, leaving an "abasic site." These sites, also known as apurinic or apyrimidinic (AP) sites, are devoid of a nucleobase but still retain the ribose-phosphate backbone. Abasic RNA modifications are often introduced in experimental settings to study the effects of missing bases on RNA stability, structure, and function. In therapeutic and molecular biology contexts, abasic sites are used to disrupt RNA duplex formation or ribonucleoprotein interactions, affecting the biological activity of the RNA. RNA molecules with abasic sites are generally less stable because the absence of a base weakens the hydrogen bonding required for proper base pairing with complementary sequences. However, abasic modifications can also be used to intentionally destabilize RNA structures for certain applications, such as regulating gene expression or studying RNA degradation pathways.
Other Modifications
- 2'-Deoxy bases
- 2'-F-arabinonucleic acid (2'-F-ANA)
- 5'-Fluorescein (Fl)
- 5' Thiophosphate
- 5'-Cholesterol (Chl)
- 5-Methyl-Deoxycytidine (5-M-Dc)
- MOE-5-Methyl-Cytidine
- 3'-Cholesterol (3'-Chl)
- 3'TEG-Cholesterol (TEG-Chl-3')
Why Choose Our CAOS Service?

Advanced Manufacturing & Quality
Precise Synthesis
Committed to providing high-precision synthesis for precise sequence-specific regulation of gene expression.
Innovative Modifications
Utilization of phosphate thioester, 2'-O-methyl, and other modifications for high nuclease resistance and lower toxicity.
Rigorous QC & Regulatory Insight
Stringent quality control from raw materials to final release. Our processes far exceed industry standards.
Collaboration Process
Consultation
Target & Goals
Design
Sequence Optimization
Synthesis
Production & QC
Delivery
Report & Support
Trusted by Global Innovators

Dr. Elena Rodriguez
Professor of Neurogenetics
"The resulting ASOs exhibited unprecedented allele specificity in our mouse models. Their follow-up support was particularly invaluable."

Dr. Michael Zhang
CEO
"Creative Biolabs delivered ahead of schedule. Their rigorous quality control provided us with the complete data package needed for investors."

Dr. Sarah Wilkinson
Senior Director, Oncology
"Their technical expertise in 4'-C-alkoxy modified nucleotides played a crucial role. The resulting ASO demonstrated exceptional potency."
Frequently Asked Questions
While standard synthetic methods can stably synthesize ASOs of approximately 100 nucleotides, the optimal and most reliable therapeutic range for highly modified ASOs (e.g., complete PS backbone and 2'-MOE) is 15 to 25 nucleotides. Longer oligomers are prone to purification difficulties.
For general in vivo studies, a purity of ≥90% is usually acceptable. However, for rigorous preclinical toxicology studies and clinical trials, a purity of ≥95% (typically ≥98%) is required to minimize toxicity.
Yes. We can provide specific chiral bond stereocontrol synthesis methods (R_p and S_p diastereomers) to optimize RNase H cleavage efficiency and safety.
Locked nucleic acids (LNAs) typically improve thermal stability (T_m) by about 2-8°C per modification, allowing for the design of shorter, more efficient ASOs.
We offer forced degradation studies (heat, acid, alkali, oxidative stress) and long-term storage assessments to predict shelf life for IND applications.
Absolutely. We handle non-standard bases (e.g., inosine, modified cytosine) and complex fluorescent dyes (Cy3, Cy5, FAM), quenchers, and special linkers.
Connect with Us for Custom ASO Synthesis
Creative Biolabs combines deep scientific knowledge with cutting-edge instrumentation and a rigorous quality system to build customized ASO synthesis services. Contact us today for more information.
Start Your Project Today
Tell us about your project, and our experts will get back to you with a customized quote and proposal.
