Cell Carrier Systems for Oncolytic Virus Delivery
Introduction of Oncolytic Virus Delivery
Oncolytic viruses have been considered as a powerful tool for treating various human diseases, especially for cancer therapy. In general, genetically engineered viruses or viruses that can utilize transformed cells have been broadly used for killing various cancer cells. These viruses can be specifically delivered to tumor burdens without damaging normal tissues and cells.
In recent years, many types of research have been designed to study the role of immune barriers in systemic oncolytic virus delivery. The data have suggested that these immune barriers are associated with poor tumor penetration, which can reduce the therapeutic efficacy of tested oncolytic viruses. As a consequence, a variety of delivery assays have been developed for transmitting the therapeutic payload of the oncolytic virus to target tumor sites by avoiding immune surveillance. For instance, the intratumoral injection has become a common method for delivering oncolytic viruses to treating different solid tumors.
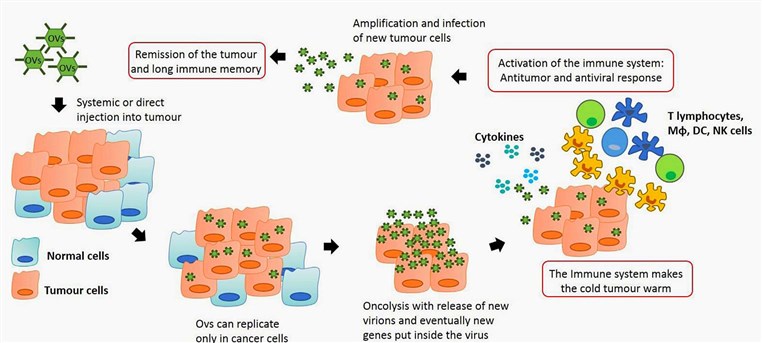 Fig.1 Anti-tumor immunity by oncolytic virus (OV) therapy. (Marelli, 2018)
Fig.1 Anti-tumor immunity by oncolytic virus (OV) therapy. (Marelli, 2018)
Cell Carrier Systems for Oncolytic Virus Delivery
In the past few years, several different oncolytic viruses have been modified and used to infect and destroy cancer cells both in preclinical and clinical trials. A series of delivery systems, such as intratumoral delivery, have been developed for carrying specific oncolytic viruses to kill tumors. However, recent results have shown that intratumoral delivery is usually transient and useless due to the rapid immune responses caused by oncolytic virus infection. In this condition, a range of cells has been identified as suitable delivery vehicles for oncolytic virotherapy. Among them, primary T lymphocytes, cytokine-induced killer (CIK) cells, mesenchymal progenitor cells, and immortal cell lines, are widely used for delivering different types of oncolytic viruses, such as adenovirus and measles virus, in the treatment of various solid tumors.
Besides, pilot studies have revealed that cell carrier systems can protect the oncolytic virus from neutralizing antibodies during the whole process of delivery, offering an effective way to improve the performance of antiviral immunity. Moreover, many kinds of cell lines, especially those derived from hematology, can serve as a comprehensive platform for the generation of therapeutic vectors to improve the efficacy of viruses in clinical use.
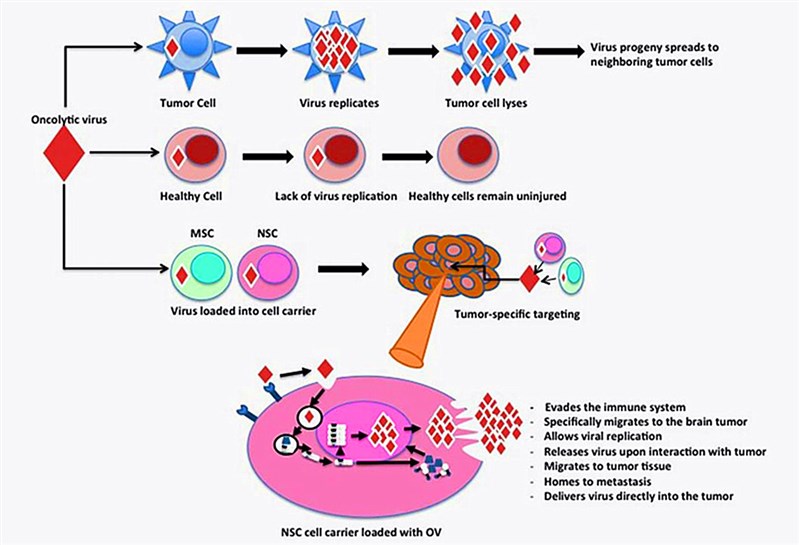 Fig.2 Comparison of modified virus behavior in healthy cells and tumor cells. (Kim, 2015)
Fig.2 Comparison of modified virus behavior in healthy cells and tumor cells. (Kim, 2015)
Cell Carrier Systems in Clinical Settings
Nowadays, many tremendous advances have been made in the development of efficient cell-based delivery of oncolytic viruses. Furthermore, many studies have been designed for clinically evaluating cell-mediated delivery of oncolytic viruses in patients with various tumor types. In general, cell carriers play an important role in regulating viral replication and in route dose amplification in oncolytic virotherapy. Also, transformed cell carriers are capable of producing thousands of virus particles per cell without causing any side reactions. Evidence has suggested that cells can not grow and lose their tumorigenic property, while still maintaining virus infection characteristics when irradiated before administration. Besides, a battery of factors, such as cell viability, cell trafficking, as well as cell effector function, should be analyzed when using carrier cells in the clinic.
References
- Marelli, G.; et al. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Frontiers in immunology. 2018, 9: 866.
- Kim, J.; et al. Stem cell-based cell carrier for targeted oncolytic virotherapy: translational opportunity and open questions. Viruses. 2015, 7(12): 6200-6217.
