Early Adenovirus Protein Controlling Services
Introduction
Creative Biolabs knows optimizing gene expression, enhancing viral vector safety, and refining targeted delivery is tough in gene therapy. Our OncoVirapy™ Platform uses advanced molecular engineering to solve these, speeding up research. We offer tailored solutions for precise early adenovirus protein expression control. Our highly characterized viral vectors have minimal immunogenicity, maximal efficacy, tight gene regulation, reduced off-target effects, and better safety for exceptional project results.
[Discover How We Can Help - Request a Consultation]
Control of Early Adenovirus Proteins
Adenoviruses are powerful tools in gene therapy and vaccine development due to their high transduction efficiency and broad tropism. Their utility, however, hinges on carefully controlling their replication and immunogenicity. Early adenovirus proteins (E1, E2, E3, E4) are pivotal in regulating viral replication, host immune response evasion, and gene expression, making their modulation essential for safe and effective therapeutic applications. Our solutions at Creative Biolabs are founded on robust research into these critical viral mechanisms.
The early proteins of adenovirus play crucial roles in orchestrating the viral life cycle, host cell manipulation, and immune evasion. Understanding and controlling these proteins is fundamental for designing safe and effective adenovirus-based vectors.
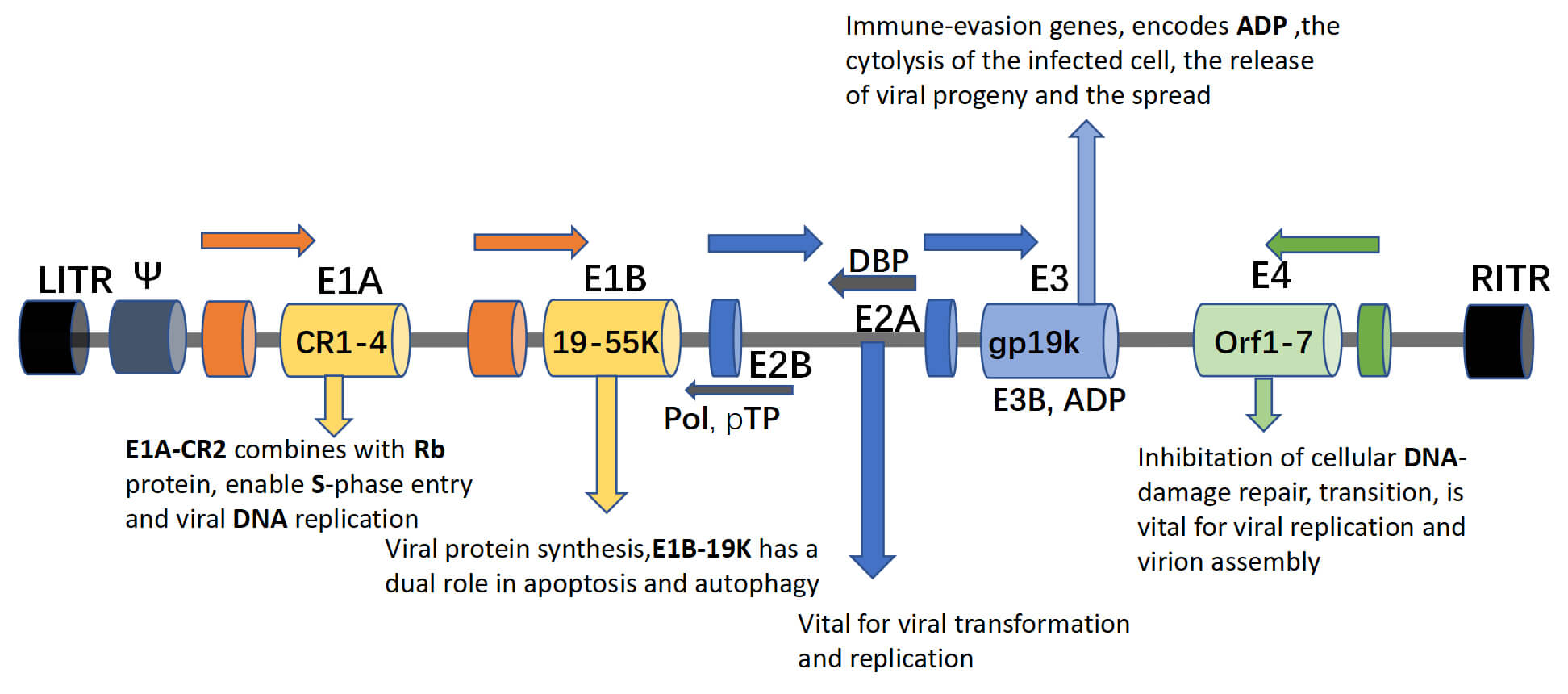 Fig.1 Components of the oncolytic adenovirus genome and their functions.1
Fig.1 Components of the oncolytic adenovirus genome and their functions.1
- E1B: E1B is vital for viral replication and immune evasion. E1B-55K and E1B-19K block E1A/p53-induced apoptosis, with E1B-55K also shuttling viral mRNAs and degrading tumor suppressors. Deleting E1B attenuates adenoviral vectors for safer gene therapy, a service Creative Biolabs specializes in with precise modifications.
- E2A: E2A, a single-stranded DNA-binding protein (DBP), is critical for adenoviral DNA replication and late gene expression. It complexes with DNA polymerase and preterminal protein to enable efficient DNA synthesis, influences viral gene transcription, and modulates the host cell cycle. Adjusting E2A expression can alter viral replication kinetics and gene delivery efficiency.
- E2B: E2B encodes the viral DNA polymerase and preterminal protein, essential for adenoviral DNA replication. The preterminal protein primes DNA synthesis, while the polymerase drives elongation, working with E2A to form the replication machinery. Modulating E2B directly impacts vector replicative capacity, crucial for designing conditionally replicative oncolytic viruses or replication-defective gene therapy vectors.
- E3: E3 proteins modulate host immune responses by inhibiting MHC class I expression, apoptosis, and TNF-α, aiding viral immune evasion. For gene therapy, minimizing E3 reduces immunogenicity for re-administration, while oncolytic viruses may retain E3 to enhance tumor lysis.
- E4: E4 proteins regulate viral gene expression, DNA replication, mRNA transport, and host cell processes. E4orf6 promotes late gene expression and inhibits host protein synthesis. Precise E4 control is vital for optimizing vector performance and safety.
Workflow
1. E1B Protein Modification Services
- Splice Isoform Regulation: Mutate the E1B gene to adjust isoform ratios, boosting oncolytic activity in cancer cells while inhibiting replication in normal cells.
- Functional Domain Engineering: Delete the p53-binding domain of E1B-55kDa to enable selective replication in p53-deficient tumors for targeted killing.
2. E2 Region Protein Services
- Promoter Replacement: Substitute E2A/B promoters with tumor-specific promoters to restrict expression in normal cells.
- Replication Efficiency Optimization: Edit E2 regulatory elements to enhance synergy among DBP, Pol, and pTP, accelerating viral genome replication.
3. E3 Region Protein Modification Services
-
Immune Balance Strategies:
- E3 Deletion: Release tumor antigens to enhance immune activation.
- E3 Retention: Preserve E3-19kDa to evade immune clearance and extend intratumoral replication.
- Immune Factor Insertion: Introduce GM-CSF/IL-12 into the E3 region to reshape the tumor microenvironment.
4. E4 Region Protein Services
- Replication Cascade Optimization: Edit E4orf6 to enhance synergy with E1B-55kDa, promoting viral mRNA nuclear export and genome packaging.
- Combination Therapy Design: Integrate E4 regulatory features with chemotherapy or CAR-T therapy to synergize oncolysis and immune activation.
5. In Vitro & In Vivo Functional Assay Services
-
In Vitro Validation:
- Replication Selectivity: TCID50 assay to measure viral titer differences between tumor cells and normal cells.
- Cytotoxicity: CCK-8/Annexin V assays for tumor cell killing efficiency and apoptosis induction.
- Immune Activation: ELISA to detect IFN-γ, TNF-α, and other cytokines released from infected tumor cells.
-
In Vivo Validation:
- Tumor-bearing Mouse Models: Monitor tumor volume, biodistribution, and survival.
- Immune Infiltration Analysis: Flow cytometry to assess CD8+ T cell, NK cell ratios, and Treg cell suppression in tumors.
6. Safety Testing Services
-
Off-Target Toxicity Assessment:
- Normal Tissue Replication: RT-PCR for viral genome copies in liver/kidney tissues.
- Hematological Toxicity: Blood routine tests and liver/kidney function (ALT/AST, creatinine) assays.
-
Immunogenicity Analysis:
- Neutralizing Antibody Detection: ELISA for anti-adenovirus antibody titers in serum.
- Cytokine Storm Risk: Plasma IL-6, IL-8, and other proinflammatory cytokine level monitoring.
7. Virus Production & Purification Services
-
Scale-Up Production:
- Serum-Free Suspension Culture: High-density HEK293 cell culture (>5×106 cells/mL) compatible with GMP-grade bioreactors.
- Batch Scale-Up: Process scaling from shake flasks to fermenters, yielding 1013 VP per batch.
-
Purification Process:
- Two-Column Chromatography: Anion exchange and size exclusion, achieving >95% purity (HPLC-verified).
- Endotoxin Control: Affinity chromatography to remove endotoxins (<1 EU/mg viral protein).
-
Formulation Development:
- Lyophilized protectant formula (trehalose and mannitol) stable at -80°C for >12 months.
- Clinical-grade filling: Aseptic lyophilized vials compliant with FDA/EMA CMC standards.
8. Customized Combination Therapy Design
- Oncolytic-Immunotherapy Synergy: E3-region IL-12 insertion combined with PD-L1 antibody increases CD8+ T cell infiltration by 2.3-fold in "cold tumor" models.
- Oncolytic-Chemotherapy Combination: E2 promoter replacement with hypoxia-responsive element (HRE) enhances viral replication efficiency by 40% in hypoxic tumors when combined with cisplatin.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
FAQs
Q: How do Creative Biolabs ensure the specificity of gene expression with its adenovirus vectors?
A: We use advanced promoter engineering and regulatory elements for highly specific target gene expression, minimizing off-target effects.
Q: What safety measures are integrated into Creative Biolabs' adenovirus protein control service?
A: We specialize in creating replication-defective adenovirus vectors by precisely deleting/inactivating early genes (E1, E3) to prevent replication in non-permissive cells. All vectors undergo strict QC for purity and sterility.
Q: Can Creative Biolabs' service be adapted for different therapeutic applications?
A: Our approach to controlling early adenovirus proteins is highly flexible. Whether your project involves gene therapy, vaccine development, or oncolytic virotherapy, we can tailor the vector design and protein expression profiles to meet your specific therapeutic goals.
Q: How does Creative Biolabs' approach compare to traditional adenovirus vector production methods?
A: Unlike traditional methods that may offer less precise control over early protein expression, Creative Biolabs utilizes advanced molecular engineering techniques for highly regulated gene modification. This results in superior vector performance, reduced immunogenicity, and enhanced safety compared to conventional approaches.
Q: What is the typical turnaround time for projects involving early adenovirus protein control?
A: The timeframe can vary depending on project complexity and specific characterization requirements, typically ranging from 8 to 16 weeks. We prioritize efficient execution while maintaining the highest quality standards.
Our service offers a robust and sophisticated solution for researchers and biopharmaceutical companies seeking to optimize viral vector performance for gene therapy, vaccine development, and oncology. By precisely modulating critical early viral proteins, we empower your projects with enhanced safety, specificity, and efficacy.
[Contact Our Team for More Information]
Related Sections
Reference
- Wu, Chuang, et al. "Tropism and transduction of oncolytic adenovirus vectors in prostate cancer therapy." Frontiers in Bioscience-Landmark 26.10 (2021): 866-872. DOI: 10.52586/4993. Distributed under Open Access license CC BY 4.0, without modification.
