Characterization of Click Chemistry Reagent
Click Chemistry Introduction
Click chemistry is a chemical synthesis method that emphasizes the efficiency, simplicity, selectivity, and modularity of chemical processes used to connect molecular building blocks. It includes the development and use of 'click to react', a set of simple biocompatible chemical reactions that meet specific criteria such as high yield, fast reaction rate, and minimal by-products. To view a response as a click response, it must meet certain characteristics:
- Modularization
- Not sensitive to solvent parameters
- High chemical yield
- Not sensitive to oxygen and water
- Regional specificity and stereospecificity
- A large thermodynamic driving force (>20kcal/mol) is advantageous for reactions with a single reaction product. An obvious exothermic reaction causes the reactants to 'spring load'.
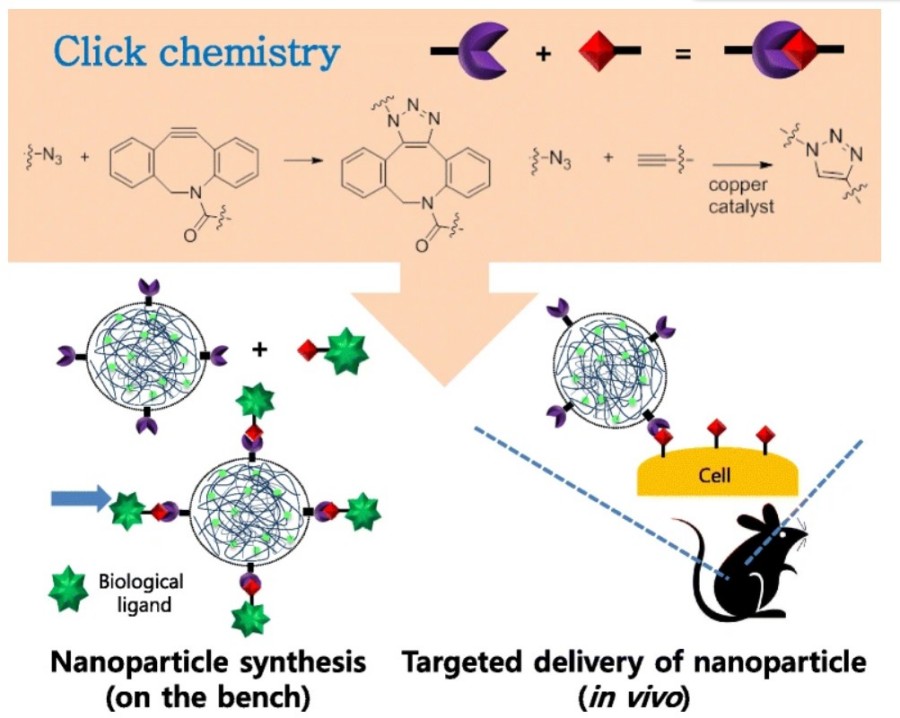 Figure 1 Illustration for the usage of click chemistry during nanoparticle synthesis and its targeting in vivo.1
Figure 1 Illustration for the usage of click chemistry during nanoparticle synthesis and its targeting in vivo.1
Click Chemistry Reagents
Click chemistry reagents cover various azides, alkynes, catalysts, and ligands, which help accelerate the exciting research in the field of click chemistry. Click chemistry refers to a chemical reaction that is modular, efficient, widely applicable, highly productive, and produces only harmless by-products. The most famous example of a "click" reaction is the copper (I) catalyzed 1,3-dipolar cycloaddition reaction of azides alkynes (CuAAC), which generates 1,4-disubstituted pentagonal 1,2,3-triazole rings.
Amino Acid Azides/Alkynes for Click Chemistry
The use of natural and non-natural amino acids for peptide synthesis is a powerful tool for developing therapies and understanding biochemistry.
Azide Source for Click Chemistry
The incorporation of azide functional groups into organic molecules is becoming an increasingly important task, as these components continue to influence organic chemistry and biology in various applications ranging from amino protection to chemical bonding.
Organic Nitrides for Click Chemistry
The azide group can also be used as a protective group for primary amines, especially in sensitive substrates such as complex carbohydrates or peptide nucleic acids (PNA) and coordination compounds, as azides are stable to olefin metathesis conditions.
PEG Azide for Click Chemistry
PEG polymers also possess many inherent favourable biological characteristics, such as high water solubility, non-toxicity and non-immunogenicity. In many cases, the chemical modification ("PEGylation") of bioactive molecules such as peptides, antibody fragments, enzymes or small molecules with polyethylene glycol chains will therefore improve pharmacokinetics and biological activity.
Characterization of Click Chemistry Reagents
Characterization is not just a purity check. It is a series of extensive, specific tests to prove that the click reagent has the correct structure, the correct function, and that it is consistent from one batch to the next. A 95% HPLC pure reagent is not valuable if the other 5% purity is of isomers or byproducts that will poison the click reaction or that will cause nonspecific binding.
Structural Integrity
The reagent contains the expected functional groups (such as terminal alkynes or DBCO groups), and there are no unexpected structural modifications (such as alkynes oxidizing to ketones). For example, DBCO reagents with damaged cyclooctyne rings will not be able to perform SPAAC reactions because the strain required for cycloaddition will be lost.
Purity
The reagent does not contain impurities that may interfere with click reactions or damage biological samples (such as unreacted starting materials, by-products, or heavy metals such as copper). A study published in Bioconjugation Chemistry in 2023 showed that even 5% residual copper in CuAAC reagents can cause non-specific binding to proteins, rendering downstream immunoassay results invalid.
Functional Activity
The reagent can effectively react with its target partner under expected conditions (e.g. physiological pH value for in vivo application). For example, the tetrazine reagent used for IEDDA must exhibit rapid reaction kinetics with trans cyclooctene (TCO) in buffer - typically a second-order rate constant (k ₂)>10 ⁴ M ⁻¹ s ⁻¹ for biomedical applications.
Characterization Methods
| Analytical Method | Primary Purpose | Key Information Provided |
|---|---|---|
| Mass Spectrometry (MS) | Identity and Molecular Weight | Exact mass, confirmation of molecular formula, detection of trace impurities. |
| NMR Spectroscopy (1H,13C) | Structure and Purity | Confirmation of chemical structure, functional group environments, non-chromophoric impurities. |
| HPLC (UV,ELSD) | Purity and Quantification | Percent purity, separation and quantification of impurities/side products, concentration. |
| Fourier-Transform Infrared (FT-IR) | Functional Group Presence | Specific vibrational modes confirming key groups, e.g., the strong, sharp N3 stretch (~2100 cm-1). |
| UV-Vis Spectrophotometry | Concentration and Molar Extinction | Quantification of chromophoric reagents (e.g., DBCO, tetrazines) and confirmation of ϵ. |
Conclusion
The characterization of chemical reagents is a crucial multi parameter process that goes far beyond basic purity analysis. It involves rigorous structural elucidation using NMR and HRMS to confirm molecular identity and regional chemistry. Chromatographic analysis (UPLC/HPLC) can identify and quantify impurities that may inhibit reactivity. It is crucial that the functional quantification of active groups (such as azides, alkynes, tetrazine) ensures predictable stoichiometry and reaction kinetics. Stability studies under various conditions provide information for proper handling and storage. At Creative Biolabs, this comprehensive representation is non-negotiable. It is the foundation for establishing reliable, efficient, and reproducible biological couplings, reducing risks in drug delivery, diagnosis, and materials science projects by ensuring that the tools you use run as expected every time.
Customer Review
Frequently Asked Questions
Q: How do you perform stability testing on Click Chemistry reagents?
A: Stability testing involves placing the material under predefined conditions, room temperature, and sometimes controllable humidity, and regularly reanalyzing its purity and functional titers using HPLC and UV Vis. For water sensitive reagents such as activated esters, monitor the hydrolysis rate in aqueous solutions of different pH values and inform customers of the optimal storage and reaction conditions.
Q: Why is NMR considered the gold standard for structure confirmation, even when HRMS data are available?
A: While HRMS provides the exact mass and formula, it does not provide information about how the atoms are connected. NMR provides a molecular connectivity map, showing which atoms are bonded and which functional groups are present. For example, NMR can distinguish between structural isomers with the same exact mass, thereby confirming the correct regio- and stereochemistry necessary for a biologically active agent.
Q: Which click chemistry reaction is best for in vivo applications?
A: SPAAC and Inverse Electron-Demand Diels-Alder (IEDDA) reaction (Tetrazine Ligation) are often preferred for in vivo applications. SPAAC is copper-free, avoiding toxicity concerns of metals, and its kinetics is fast. IEDDA is even faster (it is among the fastest bioorthogonal reactions) and forms very stable conjugates, so it is particularly well-suited for fast labeling and imaging in vivo, where speed and biocompatibility are key. CuAAC is generally avoided for in vivo applications due to copper ion toxicity.
Q: How do I choose the right spacer arm for my click chemistry reagent?
A: Selection of the appropriate spacer arm depends on the intended application:
- Solubility: If you wish to perform the reaction in aqueous media or if you want to improve the solubility of the final conjugate, hydrophilic spacers such as PEG (polyethylene glycol) chains are available.
- Steric Hindrance: Longer and more flexible spacers (e.g., longer PEG chains) can reduce steric hindrance between conjugated molecules, thus better maintaining the biological activity of the molecule.
- Targeting: In certain applications, precise distance or orientation is required between the two components and thus very short, rigid spacers may be desired.
- Cleavability: If you would like to be able to release one portion of the conjugate at a later stage, a cleavable linker can be chosen (examples include disulfide for reductive cleavage, hydrazone for pH dependent cleavage or a peptide sequence for enzymatic cleavage).
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a world class supplier of bioconjugation services and reagents. We provide complete bioconjugation support to help enable science and drug development. With years of experience in chemical biology, protein engineering and synthetic chemistry, we have a large selection of quality click chemistry reagents and services to support the unique needs of our customers. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended Products
Recommended Services
- Protein & Antibody Related Conjugation
- Nucleic Acids/Oligonucleotides Related Conjugation
- Small Molecule Related Conjugation
- Bacteria Related Conjugation
- Click Chemistry based Conjugation
- Custom Virus Conjugation
- Custom Bacteriophage Conjugation
Reference
- Yi G, Son J, Yoo J, et al. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomaterials Research, 2018, 22(1): 13. https://doi.org/10.1186/s40824-018-0123-0. Distributed under Open Access license CC BY 4.0, without modification.

