Copper Free Click Reagents
What is Copper-Free Click Chemistry?
Copper free click chemistry is a bioorthogonal reaction that is a variant of the Huisgen cycloaddition reaction of azide alkynes. By eliminating cytotoxic copper catalysts, the reaction proceeds without live cell toxicity. It was developed as a faster alternative to the first-generation copper free click chemistry Staudinger connection, producing a rate constant that is over 63 times faster. Although the reaction produces a mixture of stereoisomers of triazole, the lack of regioselectivity in the reaction is not the main issue in its application in bioorthogonal chemistry. The traditional Huisgen cycloaddition reaction can best meet the requirements of more region specificity and less bioorthogonality, especially considering the low yield and difficulty in synthesizing strain cycloalkynes (compared to the addition of terminal alkynes). The biological orthogonality of the reaction enables the copper free click reaction to be applied to cultured cells, live zebrafish, and mice.
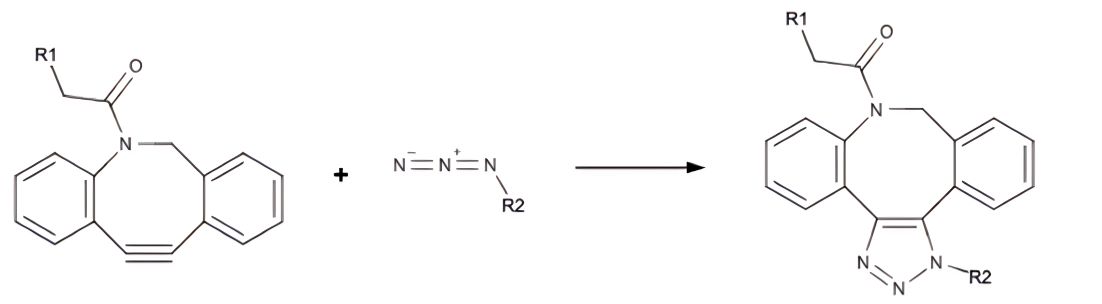 Figure 1 Cycloaddition between dibenzocyclooctyl and azide.1
Figure 1 Cycloaddition between dibenzocyclooctyl and azide.1
Copper Free Click Reagents Introduction
The copper (I) catalyzed azide alkyne cycloaddition (CuAAC) reaction between azides and alkynes reported by Sharpless forms 1,2,3-triazoles. It was found that even under the mildest conditions, this reaction is so finely regioselective and efficient that Sharpless coined the term "click chemistry" to describe it. Due to the fact that copper ions can damage DNA, often leading to strand breaks, the use of this method in DNA modification has been delayed. Due to the fact that these issues have now been overcome through the use of copper (I) stable ligands (such as tris (benzyltriazolyl) amine, TBTA3), researchers found that the CuAAC reaction can be used to functionalize alkyne modified DNA nucleobases with extremely high efficiency.
Features of Copper Free Click Reagents
- Simple to use
- Stable in solution on the synthesizer
- Stable to ammonium hydroxide and AMA
- Excellent click performance in 17 hours or less at room temperature
Development of Copper Free Click Reagents
Fluorinated Cyclooctynes
Cyclooctane derivative OCT was the first OCT developed for copper free click chemistry; It only relies on ring strain to drive the reaction forward, and the kinetics have hardly improved compared to Staudinger connections. After OCT and MOFO (monofluorocyclooctene), difluorocyclooctene (DIFO) was developed. An improved synthesis method of monofluorosubstituted cyclooctayne (MFCO) was introduced, which can be easily converted into useful reactive intermediates for bio coupling applications, although its reactivity is slightly slower than DIFO. MFCO exhibits excellent stability during long-term storage.
Fluorinated Cyclooctynes
Cyclooctane derivative OCT was the first OCT developed for copper free click chemistry; It only relies on ring strain to drive the reaction forward, and the kinetics have hardly improved compared to Staudinger connections. After OCT and MOFO (monofluorocyclooctene), difluorocyclooctene (DIFO) was developed. An improved synthesis method of monofluorosubstituted cyclooctayne (MFCO) was introduced, which can be easily converted into useful reactive intermediates for bio coupling applications, although its reactivity is slightly slower than DIFO. MFCO exhibits excellent stability during long-term storage.
Mechanism of Copper Free Click Reagents
- Cyclooctynes and Azides - In copper-free click chemistry, a cyclooctyne (e.g. DBCO, BCN) reacts with an azide to form a stable triazole ring.
- Ring Strain - The strain in the cyclooctyne ring promotes the reaction, eliminating the need for a copper catalyst.
- Bioorthogonal - The reaction is highly selective and does not interfere with other biological molecules or processes.
Spacer Arm Cleavability of Copper-Free Click Reagents
Click chemistry reagents for use in more complex bioconjugation applications, such as in antibody drug conjugates (ADCs), target identification, and release assays, are often provided with cleavable spacer arms. A spacer arm is a linker that separates the click moiety from the biomolecule or payload to which it is conjugated. Cleavable spacer arms are often used to add another controlled-release mechanism, often triggered by some physiologic or externally applied stimulus, to the bioconjugation strategy.
Frequently Asked Questions
Q: What is the benefit of copper-free click chemistry compared to CuAAC?
A: Biocompatibility. Copper-free click chemistry can be performed without a toxic copper catalyst. This makes it well-suited for in vivo applications, live-cell labeling, and reactions with sensitive biomolecules that may be inactivated or interfered with by copper ions.
Q: How fast are copper-free click reactions?
A: The original SPAAC reactions (azide-cyclooctyne) are generally slower than CuAAC. However, better reagents, especially those based on highly strained cyclooctynes (ADIBO/DIBAC) have been designed to achieve high reaction rates. In addition, the inverse electron-demand Diels-Alder (IEDDA) reaction of tetrazines with trans-cyclooctenes (TCO) is orders of magnitude faster than SPAAC and CuAAC.
Q: What functional groups are used in copper-free click reactions?
A: The most common pairing is an azide with a strained alkyne (cyclooctyne derivative). For the very fast IEDDA reaction, a tetrazine reacts with a trans-cyclooctene (TCO).
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a leading resource for all things click chemistry. In particular, we specialize in all aspects of copper-free click reagents and services. We offer a wide variety of products and services to help researchers and developers in academia and industry. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended Products
Recommended Services
-
Protein & Antibody Related Conjugation
-
Nucleic Acids/Oligonucleotides Related Conjugation
-
Small Molecule Related Conjugation
-
Bacteria Related Conjugation
-
Click Chemistry based Conjugation
-
Custom Virus Conjugation
-
Custom Bacteriophage Conjugation
Reference
- Eeftens J M, van der Torre J, Burnham D R, et al. Copper-free click chemistry for attachment of biomolecules in magnetic tweezers. BMC biophysics, 2015, 8: 1-7. https://doi.org/10.1186/s13628-015-0023-9 Distributed under Open Access license CC BY 4.0, without modification.
