Bioconjugation Reagents Applications
Fundamental Concepts of Bioconjugation
Bioconjugation is the chemical process to form a stable link between two (bio)macromolecules (for example protein, lipid, carbohydrate, nucleic acid) or between a biomolecule and a small chemical compound. Although many types of biomolecule modification have been known for many years, most simple modifications are not generally regarded as bioconjugations – the term is usually used more specifically to refer to a process in which some gain-of-function for the biomolecule is intended. For example, bioconjugation may be used to attach a fluorophore, drug or purification handle to a biomolecule, or to attach a biomolecule to a polymer support. The resulting bioconjugated complexes can be used to develop new technologies, for example in drug discovery, ligand binding assays, disease diagnosis, and high-throughput screening. There have been many recent examples of the chemical modification of biomolecules with non-natural bioorthogonal functional groups, for example azide.
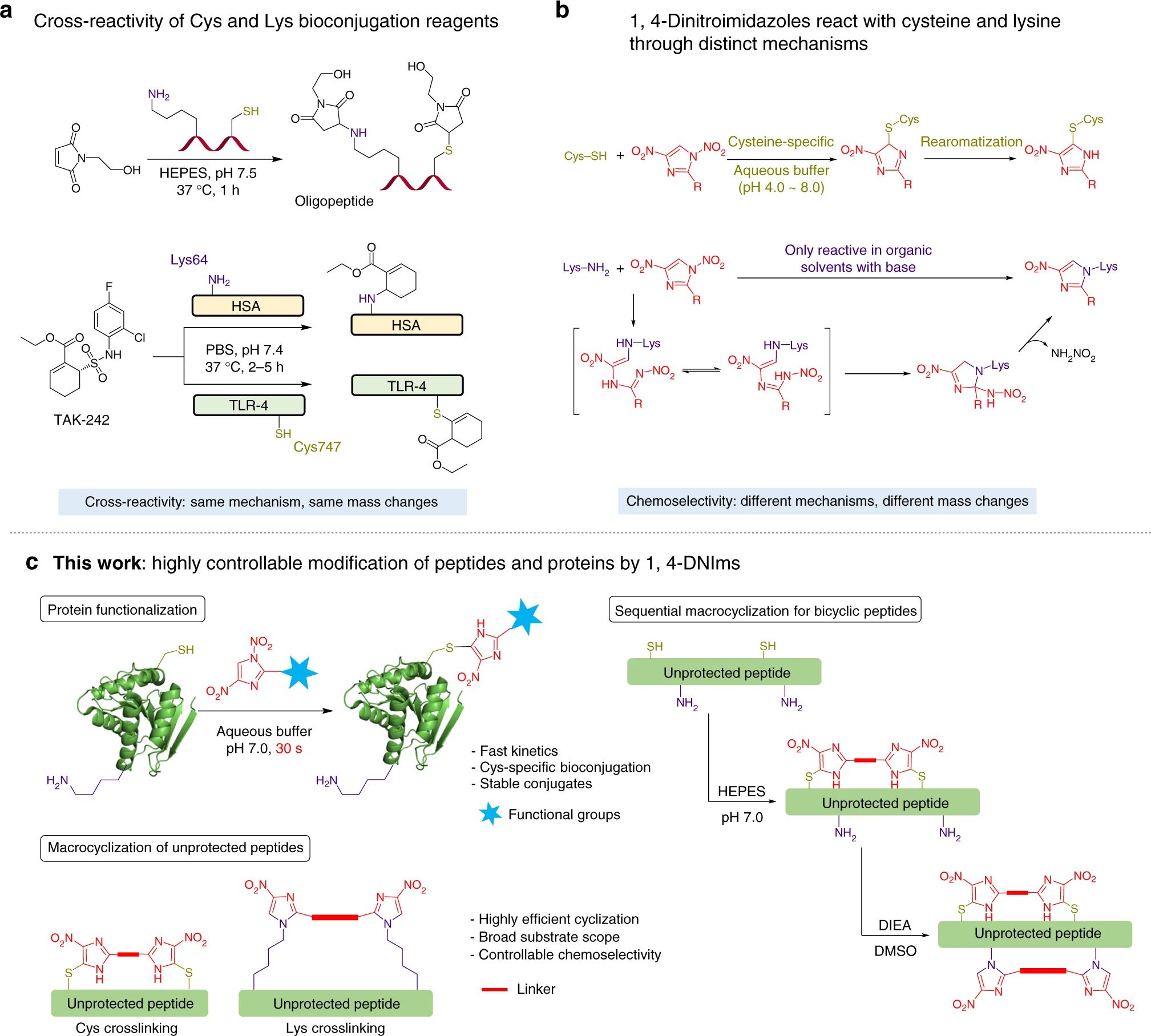 Figure 1 1,4-Dinitroimidazoles as bifunctional bioconjugation reagents.1
Figure 1 1,4-Dinitroimidazoles as bifunctional bioconjugation reagents.1
Bioconjugation Reagents Introduction
Bioconjugation reagents are essential for facilitating the covalent bonding between biomolecules and other molecules. Here is a table listing some common types of bioconjugation reagents:
| Reagent Class (Example) | Reactive Group on Reagent | Linkage Formed | Common Applications |
|---|---|---|---|
| NHS Esters (e.g., Sulfo-NHS-LC-Biotin) | N-Hydroxysuccinimide ester | Amide bond | Labeling proteins, antibodies, nucleic acids; creating amine-reactive surfaces; synthesizing active esters for further reactions. |
| Maleimides (e.g., SMCC) | Maleimide | Thioether bond | ADC synthesis, protein related conjugation, immobilization of thiolated molecules, creating thiol-reactive surfaces. |
| Hydrazides / Aldehydes (e.g., Adipic dihydrazide) | Hydrazide / Aldehyde (after oxidation) | Hydrazone bond (reducible) | Glycoprotein conjugation, carbohydrate labeling, small molecule related conjugation, specific labeling of aldehydes in biological samples. |
| Isothiocyanates (e.g., FITC) | Isothiocyanate | Thiourea bond | Fluorescent labeling of proteins and antibodies, flow cytometry, immunofluorescence. |
| Azides (e.g., Azido-PEG-NHS) | Azide (-N3) | Triazole (Click Chem) | Bioorthogonal labeling, targeted drug delivery, nanoparticle functionalization, in situ imaging, forming stable linkages in complex biological environments. |
| Alkynes (e.g., DBCO-NHS) | Alkyne (terminal or strained) | Triazole (Click Chem) | Bioorthogonal labeling, especially SPAAC (strain-promoted azide-alkyne cycloaddition) for copper-free click chemistry in live cells; similar applications to azides but offers alternative reaction kinetics. |
| Diazirines / Benzophenones | Diazirine / Benzophenone | Covalent bond | Photoaffinity labeling, studying oligonucleotides related conjugation, immobilizing biomolecules onto surfaces, crosslinking in a spatially controlled manner. |
| Pyridyl Disulfides (e.g., SPDP) | Pyridyl disulfide | Disulfide bond (reducible) | Reversible conjugation, creation of cleavable linkers for drug release, protein-protein crosslinking, and preparing reactive thiols for subsequent conjugation. |
Synthesis and Strategies of Bioconjugation Reagents
Although the chemical principle of coupling reagent reaction is simple, its implementation is not easy. Some obstacles can hinder efficient biological coupling reactions. For example, since some residues are more abundant than others, selectivity can be lacking in some reactions, and thus they are not very efficient. The other challenge is that polar molecules present on the protein surface can block the reaction. In addition, selective modification of multiple sites on proteins has also presented significant challenges. Classical protein modification strategies have primarily been based on secondary reactions that act on the side chains of certain amino acids like cysteine and lysine. Side chains of cysteine and lysine residues have thiol and amino groups, respectively, and these groups can be modified by a range of reagents.
However, with the advent of new technologies and progress in biochemistry, new strategies have been found to make biological coupling reactions more efficient. The choice of strategy largely depends on the target protein. If proteins are present in a mixture and cannot be separated, other techniques must be used. If the protein exists in purified form, the next question to consider is whether site specificity is required. Based on these initial standards, a flowchart can be used to determine effective synthesis techniques according to specific situations.
Bioconjugation Methods and Techniques
Common Bioconjugation Methods
Approaches that are commonly used to this end often incorporate a series of reagents and reactions in order to form covalent bonds between the molecules of interest. Alkylation, oxidation, and cycloaddition are several examples of techniques used in the lab.
- Alkylation, which is the process of adding alkyl groups to a molecule and changing its properties, is a common method to achieve the desired result. The method is straightforward, and is an effective and common way to treat primary amines.
- Oxidation reactions take the form of introducing oxidants to create covalent bonds. This approach is most commonly associated with thiol groups on proteins.
- Cycloaddition reactions, which include the Diels Alder reaction form covalent bonds via cyclic intermediates.
Innovative Technology of Bioconjugation
- Innovative technologies are mostly centered around enhancing the efficiency and selectivity of the biological conjugation. Click chemistry and emerging reagents have been among them.
- Clicking on chemistry (Huisgen cycloaddition reactions) can rapidly and reliably form covalent bonds between a pair of precursors. Among the various bio-orthogonal reactions, clicking chemistry has been a front-runner due to its efficiency and mild reaction conditions.
- Staudinger linkage is another widely used technology that joins phosphines with azides to form stable conjugates and is prized for its biological orthogonality. It is also compatible with live cell applications.
Advancements & the Future of Bioconjugation
In recent years, significant progress has been made in bio coupling technology, and many innovations and emerging trends are shaping its future.
Recent Innovations
The latest progress in biological coupling focuses on improving the accuracy and efficiency of drug delivery systems. Technologies such as orthogonal biological connections allow for selective and interference free modification of biomolecules, enabling more effective targeting of therapeutic drugs. Some emerging trends will affect the future of biological coupling. A significant trend is the increasing use of bio coupling in cancer treatment.
Customer Reviews
Creative Biolabs is proud to be a trusted partner in bioconjugation research. Here are two testimonials from our valued customers:
"As a lead scientist in oncology research, the purity and reactivity of bioconjugation reagents are paramount to our success. Creative Biolabs consistently provides exceptionally high-quality products that streamline our ADC development. Their custom synthesis service is a game-changer for our unique project needs."
— Dr. Anya Sharma, Lead Scientist.
"We rely heavily on precise bioconjugation for our novel diagnostic kits. The technical support from Creative Biolabs has been outstanding, helping us troubleshoot complex conjugation challenges and optimize our protocols. Their broad catalog of reagents has significantly accelerated our R&D cycle."
— Professor Liam O'Connell, Director of Diagnostics Innovation.
Overview of What Creative Biolabs Can Provide
Creative Biolabs believes that a deep understanding of bioconjugation reagents and their applications is crucial for accelerating biomedical discoveries. At Creative Biolabs, we are dedicated to providing the high-quality resources and expertise necessary to empower your research. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended products
Reference
- Luo Q, Tao Y, Sheng W, et al. Dinitroimidazoles as bifunctional bioconjugation reagents for protein functionalization and peptide macrocyclization. Nature communications, 2019, 10(1): 142.https://doi.org/10.1038/s41467-018-08010-2. Distributed under Open Access license CC BY 4.0, without modification.
