Selection Guide for Bioconjugation Reagents
Bioconjugation Basics
Biological coupling is an emerging research field. New methods for mild and site-specific derivatization of proteins, DNA, RNA, and carbohydrates have been developed for applications such as ligand discovery, disease diagnosis, and high-throughput screening. The existence of these powerful methods is attributed to the discovery of chemical selective reactions that can undergo biological coupling under physiological conditions, which is a great achievement of modern organic chemistry.
Biological coupling must have:
- Requires high concentration, high purity, and high-quality starting compounds
- High reaction level, minimal energy input, minimal by-products, and highest yield
- Need to target specific locations
- The functional group must be accessible
- Appropriate connectors must be used
- In many cases, biological coupling must be carried out with complete cellular processes
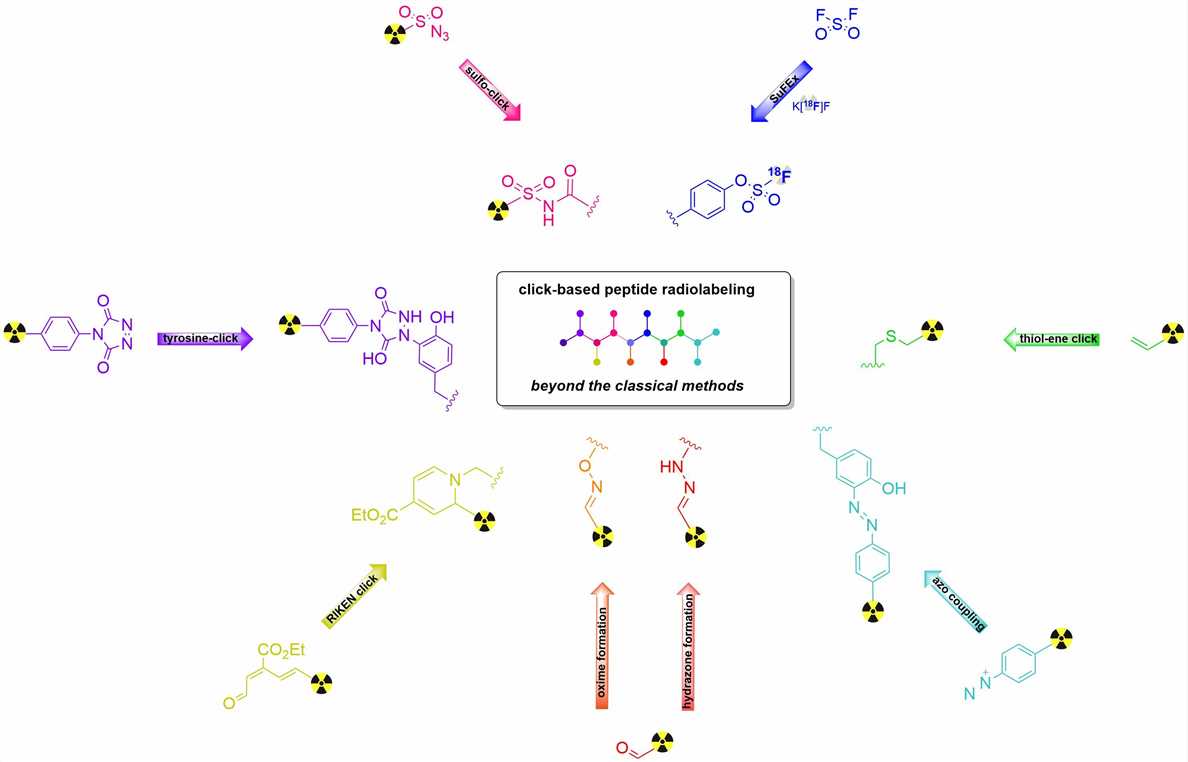 Figure 1 Innovative peptide bioconjugation.1
Figure 1 Innovative peptide bioconjugation.1
Common Bioconjugation Reactions
Synthesis of biological conjugates involves various challenges, ranging from the simple and non-specific use of fluorescent dye labeling to the complex design of antibody drug conjugates. Various biological coupling reactions have been developed to chemically modify proteins. The common biological coupling reactions on proteins include the coupling of amino acid residues such as lysine, cysteine, and tyrosine, as well as the modification of tryptophan residues and the N-terminus and C-terminus. However, these reactions often lack chemical selectivity and efficiency as they rely on the presence of natural amino acids, which in turn hinder selectivity. People are increasingly in need of chemical strategies that can effectively attach synthetic molecular sites specifically to proteins. One strategy is to first install a unique functional group on the protein, and then use bioorthogonal reactions to couple the biomolecule with this unique functional group. Bioorthogonal reactions targeting non-natural functional groups have been widely applied in bio coupling chemistry. Some important reactions include the modification of ketones and aldehydes, Staudinger linkage with organic azides, copper catalyzed Huisgen cycloaddition of azides, and strain promoted Huisgen cycloaddition of azides.
Bioconjugation Reagents
The traditional strategy of covalent biological coupling eliminates control over the reaction zone chemistry, resulting in heterogeneous reaction products. Poor control of modification sites often leads to the loss of biological function of target biomolecules. In contrast, the new biological coupling method has high site specificity and causes minimal interference to the active form of biomolecules. In addition, biomolecule fixed sites have higher ligand binding ability with specificity. Therefore, site-specific biological coupling is more desirable than random biological coupling. The common linkage of site-specific biological conjugation depends on cysteine or lysine residues. Newer methods target non-natural functional groups, including olefins through metathesis.
![Figure 2: Tyrosine-Click Bioconjugation of [18F] FS-PTAD with L-Tyrosine. (OA Literature)](static/img/4-4-1-selection-guide-for-bioconjugation-reagents-2.jpg) Figure 2 Bioconjugation of [18F] FS-PTAD with L-tyrosine via tyrosine-click.1
Figure 2 Bioconjugation of [18F] FS-PTAD with L-tyrosine via tyrosine-click.1
Table of Bioconjugate Chemistry Reagents
| Reagent Class | Functional Group | Target Biomolecule Feature | Example Reagents |
|---|---|---|---|
| Amine-reactive | NHS ester, isothiocyanate | Primary amines (-NH₂) in lysine | Sulfo-NHS, EDC, fluorescein isothiocyanate (FITC) |
| Thiol-reactive | Maleimide, iodoacetamide | Free thiols (-SH) in cysteine | SMCC, Traut's reagent, pyridyl disulfide |
| Carboxylic acid-reactive | Carbodiimide (EDC) | Carboxylic acids (-COOH) in aspartate/glutamate | EDC + NHS |
| Carbonyl-reactive | Hydrazide, aminooxy | Aldehydes/ketones (from oxidation of carbohydrates) | Hydrazide-PEG-NHS, aminooxy-SS-amine |
| Photoreactive | Azide, benzophenone | Non-specific (covalent upon UV activation) | Sulfo-SANPAH, NHS-aryl azide |
| Click chemistry | Azide, alkyne | Complementary click partner (e.g., azide-alkyne) | DBCO-PEG-NHS, azide-PEG-maleimide |
Why PEG Linkers Matter?
PEG linkers are chemically functionalized polyethylene glycol (PEG) linkers. PEG linkers are particularly attractive in the scientific community as powerful tools for conjugation, drug discovery, nanoparticle drug delivery, chemical biology, and biomarkers due to their water solubility and non-immunogenicity. There are two types of PEG, monodisperse and polydisperse. Monodisperse PEG linkers have a precise number of PEG units, specific chemical structures, and precise molecular weights. Poly dispersed PEG (also known as polymer PEG or PolyPEG) is a polymer with an average molecular weight.
PEG in Nanoparticle Drug Delivery
With the development of COVID-19 vaccine, drug delivery of lipid nanoparticles has now become famous. Based on the successful improvement of systemic circulation time and reduction of immunogenicity by PEGylated proteins, the impact of PEG coating on the fate of systemically administered nanoparticle formulations has been and will continue to be extensively studied. Typically, delivery systems consist of high transition temperature phospholipids, PEG lipids, and ionizable cationic phospholipids. The PEG coating on nanoparticles protects the surface from aggregation, conditioning, and phagocytosis, prolonging systemic circulation time.
PEG in Small Molecule Drug
Introduction of PEG linkers to small molecule drugs increases the drug's solubility and molecular weight. This may allow for an extended drug half-life in the body.
(1) Stronger biological activity;
(2) Liposomes have a stronger passive targeting effect on tumors;
(3) longer half-life;
(4) lower maximum blood concentration;
(5) minor fluctuations in plasma concentration;
(6) less enzymatic degradation;
(7) Less immunogenicity and antigenicity;
(8) less toxic;
(9) Better solubility;
(10) reduce the frequency of medication;
(11) Improve patient compliance, improve quality of life, and reduce treatment costs.
Common Types of PEG Linkers for Bioconjugation
| PEG Linker Category | Reactive End Groups / Examples | Architecture | Typical Applications |
|---|---|---|---|
| Homobifunctional PEGs | NHS-PEG-NHS, Maleimide-PEG-Maleimide, Aldehyde-PEG-Aldehyde, Amine-PEG-Amine | Linear | Crosslinking two identical molecules (e.g., proteins, peptides), forming polymeric structures or hydrogels. |
| Heterobifunctional PEGs | NHS-PEG-Maleimide, NHS-PEG-Hydrazide, Maleimide-PEG-Alkyne, NHS-PEG-Alkyne, Biotin-PEG-NHS | Linear | Directed conjugation between two different molecules (e.g., antibody to drug, protein to nanoparticle), minimizing self-conjugation. Useful for creating defined conjugates with specific orientations. |
| Multi-arm PEGs | 4-arm PEG-NHS, 8-arm PEG-Maleimide | Branched/Star-shaped | Creating highly branched or multivalent conjugates, increasing drug loading capacity, enhancing avidity for targets, forming complex hydrogels or scaffolds. |
Choosing the Right Reagent: Best Practices
Choosing the best bio coupling reagent is a multifactorial decision that has a crucial impact on the success of any bio coupling project. A systematic approach is essential considering the properties of biomolecules, the expected characteristics and applications of conjugates. The following are the best practices for selecting the correct reagents:
Characterize Biomolecules
- Stability Profile: Determine the pH, temperature, and solvent compatibility of your biomolecules. Many proteins are sensitive to extreme pH or organic solvents. Choose reagents that react efficiently under mild, aqueous conditions (e.g., physiological pH 6.5-8.5, room temperature).
- Molecular Weight and Size: Larger molecules may tolerate more extensive modification without significant loss of activity, while smaller peptides might be more sensitive.
Evaluate Reaction Chemistry Options
Prioritize the selection of chemical substances with high specificity for the desired functional groups to minimize unnecessary side reactions and improve product uniformity. Orthogonal click chemistry in biology, such as azide alkynes and tetrazine linkages, provides unparalleled specificity for pre-engineered biomolecules.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a global leader in providing comprehensive solutions for bioconjugation, serving the diverse needs of academic research, biotechnology, and pharmaceutical industries. Our expertise spans the entire spectrum of bioconjugate development, from reagent supply to custom conjugation services and analytical characterization. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended products
Reference
- Leier S, Wuest F. Innovative Peptide Bioconjugation Chemistry with Radionuclides: Beyond Classical Click Chemistry. Pharmaceuticals, 2024, 17(10): 1270. https://doi.org/10.3390/ph17101270 . Distributed under Open Access license CC BY 4.0, without modification.
