Intramolecular Click Reagent
Intramolecular Click Chemistry Basics
Click Chemistry is one of the modular, fast, and most reliable tools for the regioselective 1,2,3-triazole formation [3+2] reaction of organic azides and pteronyl alkynes, which has been widely explored in various emerging research fields from chemical biology to catalysis, pharmaceutical chemistry to materials science. This region selective reaction from various azide alkyne scaffolds has been well demonstrated both between and within molecules. Compared to the intermolecular metal (Cu/Ru/Ni) variants of click chemistry, intramolecular click tools are rarely mentioned. Intramolecular click chemistry is a modern cyclization tool that involves metal catalyzed (CuAAC/RuAAC) cyclization, organic catalyzed cyclization, and thermally induced topological chemical reactions.
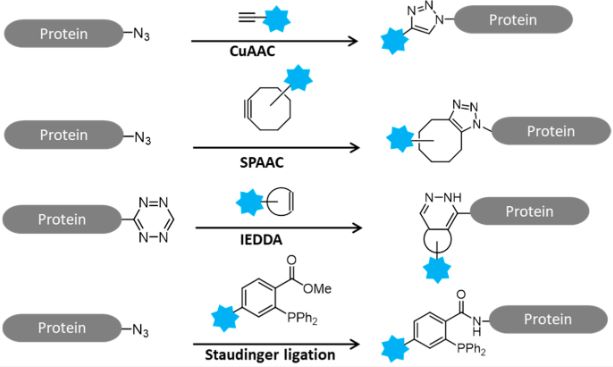 Figure 1 Schematic reactions of click chemistry.1
Figure 1 Schematic reactions of click chemistry.1
Click Reactions
The Cu (I) catalyzed 1,3-dipolar cycloaddition reaction of azides alkynes (CuAAC), commonly known as the "click reaction," is the most effective and reliable tool for easily constructing simple to complex structures at the molecular level. Over the past 15 years, the click weaving of two completely different molecular entities has created some very interesting structures with broad applicability, including in fascinating fields such as synthetic chemistry, medicine, biochemistry, pharmacology, materials science, and catalysis. The unique properties of carbohydrates and the advantages of highly chemical and regioselective click chemistry, such as mild reaction conditions, efficient performance in multiple solvents, and compatibility with different functions, have collectively produced miraculous new sugar conjugates and new sugar polymers with various synthetic, biological, and pharmaceutical applications.
What Is Intramolecular Click Reagent?
An Intramolecular Click Reagent is a chemical compound that is designed to participate in a click reaction in an intramolecular fashion; that is, as a reaction between two functional groups attached to the same chemical scaffold, rather than two free standing molecules. In this case the intramolecular (usually a cycloaddition) reaction results in the formation of a cyclic structure within the molecule itself. (See also intermolecular click reactions where the azide and alkyne are on different molecules.)
Spacer Arm Structure and Solubility of Intramolecular Click Reagent
The design of intramolecular click reagents requires careful consideration of the "spacer arm" that links the two reactive moieties. The structure of the spacer arm, including its chemical composition, length, flexibility, and branching, can have a significant impact on the efficiency of the intramolecular reaction and the properties of the resulting cyclized product. The spacer arm is not simply a passive linker; its characteristics play an active role in the reaction process and can influence both reaction rate and selectivity. This section will discuss various considerations when choosing or designing a spacer arm for intramolecular click chemistry.
Spacer Arm Structure of Intramolecular Click Reagent
If the spacer arm is short and rigid, the reactive groups will not be able to attain the proper geometry for a reaction to occur, leading to inefficiency or steric clashes. On the other hand, if the spacer arm is long and flexible, this will come at an entropic cost of cyclization, also leading to lower effective molarity and intermolecular reaction pathways if higher concentrations are used. Exact lengths will vary by system and are often determined by experimentation or modeling, but usually fall in the range of a few angstroms to several nanometers. For example, with intramolecular CuAAC, a spacer that places the azide and alkyne in close proximity and able to adopt the geometry of the transition state is required. SO it is widely used in industrial applications.
Solubility of Intramolecular Click Reagent
Intramolecular click reagents must be soluble in the reaction medium, which is often aqueous biological media. The general solubility is additive from the two reactive groups, and more importantly, the spacer arm. A general strategy for improving aqueous solubility is to integrate hydrophilic units into the spacer arm, such as PEG, oligo-saccharides, or charged units (carboxylates, amines, etc.). For instance, a DBCO-PEG-azide intramolecular reagent will be orders of magnitude more soluble in a buffer than its non-PEGylated analog. This can allow for use in a cell culture or in vivo setting without harsh organic co-solvents, which would denature or disrupt other biomolecules.
Customer Review
"We recently utilized Creative Biolabs' custom-synthesized intramolecular SPAAC reagent for the cyclization of a novel peptide antagonist targeting a GPCR. The design process was seamless, with their expert team providing invaluable input on linker length and hydrophilicity. The reagent arrived promptly, and its performance was exceptional. We achieved a remarkable cyclization yield of over 95% under mild, physiological conditions, which was critical for maintaining the peptide's activity. This level of purity and efficiency significantly accelerated our drug discovery pipeline. Creative Biolabs' commitment to quality and their deep understanding of bioconjugation chemistry are truly outstanding."
From Dr. Anya Sharma, Principal Investigator
"For our new diagnostic platform, we needed a robust and highly specific intramolecular click reaction to assemble a self-reporting biosensor. Creative Biolabs provided us with a range of their pre-made inverse electron demand Diels-Alder (IEDDA) intramolecular reagents. The speed and bioorthogonality of the tetrazine-TCO system were exactly what we needed. We were particularly impressed with the detailed product documentation and the consistent batch-to-batch quality, which is essential for our large-scale production. Their technical support team was also incredibly responsive when we had a question about optimizing reaction conditions. Creative Biolabs is now our go-to supplier for advanced bioconjugation tools."
From Mr. David Lee, Lead Scientist
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a premier biotechnology company that offers a wide range of services focused on bioconjugation and more. Built on a foundation of knowledge in synthetic organic chemistry, biochemistry, and molecular biology, Creative Biolabs provides a variety of products and services to support the ever changing and varied needs of both academic and industrial researchers. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended Products
Recommended Services
-
Protein & Antibody Related Conjugation
-
Nucleic Acids/Oligonucleotides Related Conjugation
-
Small Molecule Related Conjugation
-
Bacteria Related Conjugation
-
Click Chemistry based Conjugation
-
Custom Virus Conjugation
-
Custom Bacteriophage Conjugation
Reference
- Yao T, Xu X, Huang R. Recent advances about the applications of click reaction in chemical proteomics. Molecules, 2021, 26(17): 5368. https://doi.org/10.3390/molecules26175368Distributed under Open Access license CC BY 4.0, without modification.
