Molecular Properties of Bioconjugation Reagents
Bioconjugation Technology Introduction
Bioconjugation technology is the key to realizing well-defined and controllable covalent linkages between two or more biomolecules, which in turn enables the creation of new structures with novel functions. Because most biomolecules of interest are complex (such as proteins) and only soluble and active in aqueous media, the preparation of such bioconjugates must be made in aqueous solution and any appropriate bio-coupling chemistry must be compatible with the biological activity and function of biomolecules in such an environment. Conjugates are typically formed by introducing separate, but complementary, functional groups to each of two biomolecules. These functional groups are then typically introduced through a process known as modification, whereby linkers are attached to amines or thiols present on the biomolecule of interest. The two modified biomolecules are then mixed, and the desired biological conjugate is formed via the complementary linkers that were introduced during the modification process.
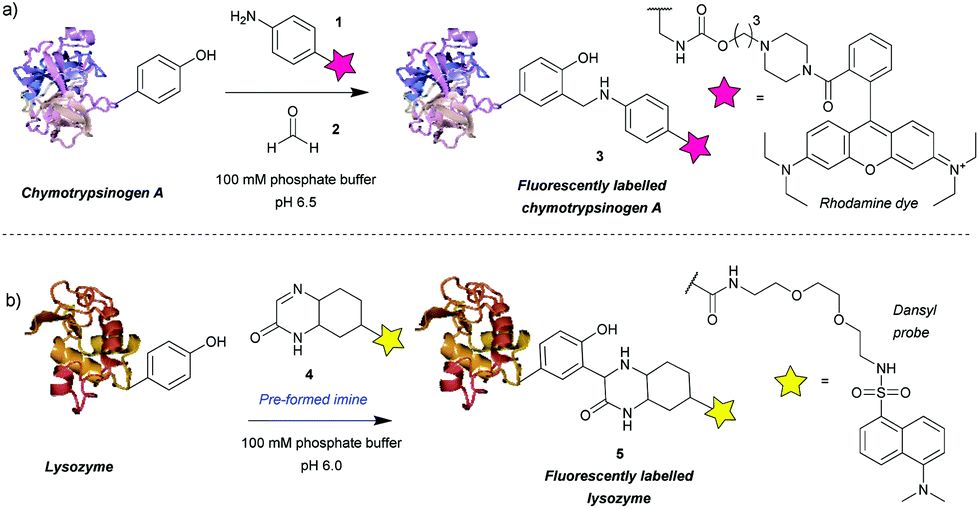 Figure 1 Tyrosine bioconjugation via Mannich-type reactions.1
Figure 1 Tyrosine bioconjugation via Mannich-type reactions.1
Specificity and Selectivity of Reactive Groups
The specificity and selectivity of cross-linking reagents is largely a function of their reactive groups that target certain functional groups of biomolecules. This specificity is important to minimize off-target effects, maintain the activity of the biomolecule, and to ensure that the desired conjugated structure is obtained.
- Overview of Reactive Groups and Their Target Functional Groups
Crosslinking reagents are broadly classified by the functional groups they react with:
| Reactive Group Type | Target Functional Group | Reaction Mechanism/Characteristics | Example Reagents |
|---|---|---|---|
| Amine-Reactive Reagents | Primary Amines (ϵ-lysine residues, N-terminus) | Form stable amide or thiourea bonds; NHS esters are reactive at pH 7-9. Isothiocyanates are less stable. | NHS Esters (e.g., Sulfo-NHS-LC-Biotin, NHS-PEG), Isothiocyanates (e.g., FITC) |
| Thiol-Reactive Reagents | Sulfhydryl Groups (cysteine residues) | Form stable thioether bonds (maleimides) or reversible disulfide bonds (pyridyl disulfides); Maleimides react quickly and selectively at pH 6.5-7.5. | Maleimides (e.g., SMCC, PEG-Maleimide), Pyridyl Disulfides (e.g., SPDP) |
| Carboxyl Activators | Carboxyl Groups (aspartic acid, glutamic acid residues, C-terminus) | Activate carboxyl groups to react with primary amines, forming amide bonds; EDC forms an O-acylisourea intermediate, which can be stabilized by NHS. | EDC/NHS System (1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide / N-Hydroxysuccinimide) |
| Click Chemistry Reagents | Azides, Alkynes | Display excellent bioorthogonality, high efficiency, and mild reaction conditions; Form stable triazole linkages. | Azides and Alkynes |
- Importance of Selecting Appropriate Reactive Groups
The choice of reactive groups has an impact on conjugation efficiency and the functionality of the conjugation. For instance, using amine reaction reagents for conjugating proteins with highly conserved lysine residues in the active site can inactivate the protein, while thiol reaction reagents with non-essential cysteine residues can be used to retain activity. Likewise, some biological orthogonal click reaction reagents are better suited to in vivo applications to avoid cross-reactivity with biological systems. Choosing the appropriate reactive groups can ensure site specificity, high efficiency, and compatibility with biomolecule structure and function in the coupling.
Length and Flexibility of Linkers
Length: Avoiding Steric Hindrance
The linker also must be of an appropriate length to prevent steric hindrance of conjugated molecules. For instance, when large biomolecules (e.g. antibodies) are conjugated to other molecules (e.g. drugs or fluorescent groups), short linkers can bring binding partners too close to each other and disrupt their natural conformation or sterically block their binding site. For example, when applied to ADCs, a too short adapter could sterically hinder the binding of antibodies to their antigen or the binding between drugs and their targets. Long linkers (e.g. 10-30 atoms long) can provide enough space for each conjugated molecule to function relatively independently and preserve its biological activity.
Flexibility: Avoiding Aggregation and Immunogenicity
Flexible linkers that allow for rotational and translational motion of the conjugated molecules (e.g. polyethylene glycol (PEG), alkyl chains) reduce intermolecular interactions that can lead to aggregation (frequent in protein conjugates), thus increasing solubility and activity.
Hydrophilicity/Hydrophobicity: Solubility and Non-Specific Binding
The polarity of the linker will have an impact on the solubility of the conjugate as well as the interaction with the biological environment.
- Solubility: Hydrophilic linkers like PEG and sulfonic acid groups increase the water solubility of conjugates. This is important for most biological applications, particularly when working in aqueous buffer systems.
- Nonspecific binding: Hydrophobic linkers can increase the possibility of non-specific interactions with a cell membrane or other proteins, which can cause increased background signal in detection or rapid clearance in vivo. Hydrophilic linkers can decrease the likelihood of this non-specific binding.
Importance of Water Solubility for Bioconjugation Reactions
Most biomolecules are stable and functional in aqueous environments. Therefore, crosslinking reagents must be sufficiently water-soluble to ensure homogeneous reaction mixtures and efficient conjugation. Poor solubility can lead to aggregation, reduced reactivity, and inconsistent results.
Factors Affecting Solubility
- Hydrophilic Groups: The incorporation of polar, water-soluble groups such as sulfonate (SO3−), polyethylene glycol (PEG), or charged amino acids significantly enhances the aqueous solubility of crosslinking reagents. For example, sulfo-NHS esters are water-soluble versions of their non-sulfonated counterparts, making them ideal for reactions in physiological buffers.
- Molecular Weight: Generally, higher molecular weight reagents, especially those with hydrophobic moieties, tend to have lower solubility unless compensated by the inclusion of hydrophilic elements.
Reagent Stability During Storage and in Reaction Buffers
- Storage Stability: Crosslinking reagents, particularly those with highly reactive functional groups like NHS esters or maleimides, are susceptible to hydrolysis or degradation over time. They are often supplied as lyophilized powders and should be stored under desiccated conditions at low temperatures (e.g., −20∘C or −80∘C) to maintain their reactivity.
- Stability in Reaction Buffers: Once dissolved in an aqueous buffer, the reactive groups can undergo hydrolysis, competing with the desired conjugation reaction. The rate of hydrolysis is often pH-dependent; for instance, NHS esters are more prone to hydrolysis at higher pH, while maleimides can undergo ring opening at very high pH. Therefore, selecting appropriate buffer conditions and reaction times is crucial to maximize conjugation efficiency and minimize reagent degradation.
Cleavable vs. Non-Cleavable Linkers
The choice between cleavable and uncut connectors depends on the application's requirements for conjugate stability and payload release.
01 Application-Driven Choice of Linker Type
The cleavable linker is designed to break under specific conditions, releasing conjugated molecules (such as drugs or probes) at the target site. This is crucial in applications such as ADC, where drugs must remain attached to antibodies during circulation but be released within cancer cells to be effective. Non cleavable linkers form permanent covalent bonds, ensuring long-term stability - ideal for applications such as diagnostic imaging, where conjugates must remain intact to signal the target.
02 Cleavable Linkers: Conditions for Cleavage
Separable connectors are classified according to their cutting triggering factors:
- PH sensitive linkers (such as hydrazones and acetals) hydrolyze in acidic environments (pH 4-5), such as the endosomes or lysosomes of cells. This is widely used in ADCs, where conjugates are internalized by cancer cells and release drugs in acidic lysosomal environments.
- Enzyme cleavable linkers (e.g. peptide-based linkers) are cleaved by specific enzymes (e.g. tissue proteases in tumors). This provides high specificity as cleavage only occurs in tissues expressing the enzyme, thereby reducing off target toxicity.
- Reductive cleavable linkers (such as disulfides) are cleaved by abundant reducing agents such as glutathione in the cytoplasm. This is very useful for the intracellular release of payloads.
- Optically cleavable connectors will break when exposed to light (such as ultraviolet or near-infrared), enabling spatiotemporal control of release - which is valuable in research applications that require precise conjugation reversal time.
03 Non-Cleavable Linkers
Unstoppable linkers form stable bonds (such as amides, thioethers) that resist cleavage under physiological conditions. They are used for applications that require long-term stability, such as fluorescently labeled antibodies for immunohistochemistry (where the label must remain attached to the antibody during staining and imaging) or fixed proteins in biosensors (where the protein must remain bound to the sensor surface for consistent detection).
Steric Hindrance Effects
The physical dimensions and three-dimensional conformations of both the crosslinking reagent and the biomolecules being conjugated can significantly influence the efficiency of the bioconjugation reaction and the functional integrity of the resulting conjugate. These are collectively known as steric hindrance effects.
Influence of Reagent Size and Shape on Reaction Efficiency
- Accessibility of Reactive Sites: Bulky crosslinking reagents might not be able to reach the reactive functional groups of the biomolecules, especially if they are located in deep pockets or sterically hindered regions. This can result in slow reaction rates or incomplete conjugation.
- Conformational Changes: The attachment of a bulky crosslinking reagent or the conjugation with a large biomolecule can induce unfavorable conformational changes in the target biomolecule. This may lead to loss of the native structure, binding affinity, or enzymatic activity.
- Reaction rate: Steric hindrance can slow down the reaction kinetics by impeding the close approach and proper orientation of the reactive groups.
Consideration of Reagent and Biomolecule 3D Structures
- Reagent design: When designing or selecting crosslinking reagents, the molecular size and shape of the reagents should be taken into account. Smaller and more linear reagents may provide better accessibility to crowded reactive sites.
- Biomolecule structure: Knowledge of the three-dimensional structure of the biomolecule (e.g., from X-ray crystallography or NMR data) can help predict potential steric hindrance issues. For example, if a reactive lysine residue is buried deep within the active site of a protein, a bulky crosslinker may not be suitable.
- Linker choice: As mentioned earlier, the use of a sufficiently long and flexible linker can help to overcome steric hindrance by providing enough space between the conjugated molecules, allowing them to maintain their independent functions.
Overview of What Creative Biolabs Can Provide
Bioconjugation is a topic we are very passionate about at Creative Biolabs and we dedicate a significant amount of time and resources in assisting you in the effective implementation of this strategy. With many years of combined experience in chemical synthesis and biomolecular engineering, we provide an extensive catalog of high-quality crosslinking reagents, as well as bioconjugation services. Our reagents are fully characterized for purity, reactivity and stability, to provide you with the most reliable and reproducible performance for your most demanding applications. We have a large catalog of reagents which includes high specific amine- and thiol-reactive reagents, click chemistry tools, and a variety of linker chemistries (cleavable and non-cleavable, hydrophilic and hydrophobic). Please feel free to contact us for a consultation and custom synthesis services. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended products
Reference
- Szijj P A, Kostadinova K A, Spears R J, et al. Tyrosine bioconjugation–an emergent alternative. Organic & Biomolecular Chemistry, 2020, 18(44): 9018-9028. https://doi.org/10.1039/D0OB01912G.Distributed under Open Access license CC BY 4.0, without modification.
