Quality Control of Crosslinker
Crosslinker Overview
Crosslinking is a method of connecting one polymer chain to another, typically through covalent or ionic bonds. These connections exist in nature (such as between proteins in the human body), but can also be applied to coating engineering processes. When polymers are initially made, they are usually elastic bodies with high viscoelasticity and weak intermolecular forces. Crosslinking makes this substance more durable and enhances its potential applications. Traditional crosslinking agents include aziridines, isocyanates, and melamine, but in recent years, new technologies such as polycarbodiimide have emerged to make crosslinking more effective in different environments.
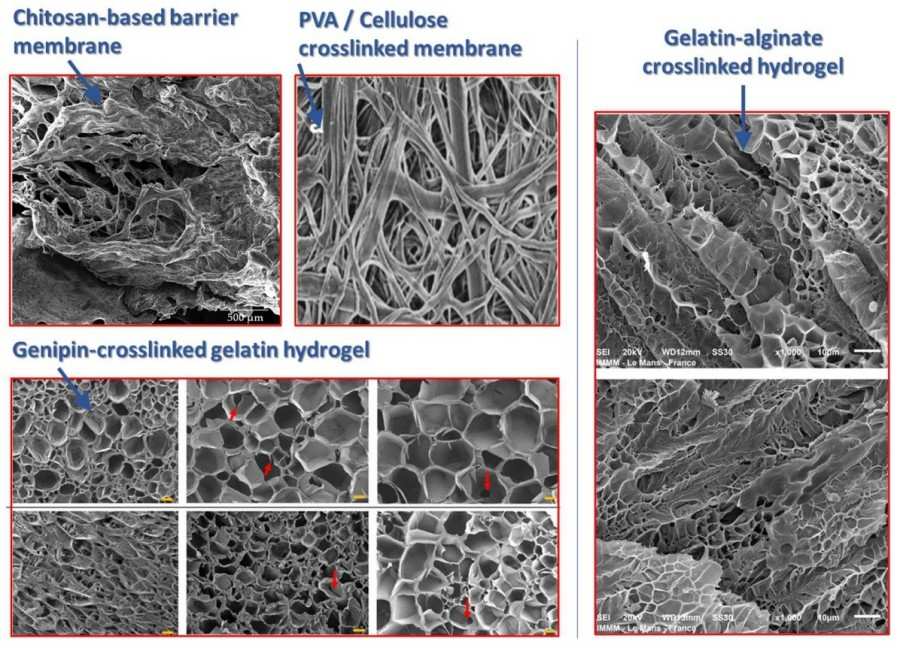 Figure 1 SEM images for different biomembranes made using different biomaterials (chitosan, cellulose, alginate, gelatin, etc.) and crosslinked by various cross-linkers (genipin, glutaraldehyde, etc.) to highlight the microstructure organization after the crosslinking process.1
Figure 1 SEM images for different biomembranes made using different biomaterials (chitosan, cellulose, alginate, gelatin, etc.) and crosslinked by various cross-linkers (genipin, glutaraldehyde, etc.) to highlight the microstructure organization after the crosslinking process.1
Crosslinkers: Chemistry and Classification
Crosslinkers are classified based on the reactivity of their functional groups. The choice of crosslinking agent depends on the functional groups present on the molecules to be connected. The following table lists common reactive groups and their target functions.
Homobifunctional Crosslinkers
containing two identical reactive groups (e.g. two NHS esters, two maleimides). They react with the same target functional groups (such as amines) on two molecules, making them ideal choices for crosslinking identical or similar biomolecules.
Heterobifunctional Crosslinkers
containing two different reactive groups (e.g. NHS ester+maleimide). They react with two different functional groups (e.g. amine on one molecule, thiol on the other molecule) to enable site-specific binding of different biomolecules (e.g. antibody drugs, protein fluorophores).
Multifunctional Crosslinkers
containing three or more reactive groups (such as NHS ester, maleimide). They form branch networks that can be used in biomaterial engineering (such as hydrogel cross-linking) or to create multi protein complexes.
Advanced Methods for Quality Control of Crosslinkers
| QC Method | Primary Analytical Purpose | Key Specification & Analytical Detail |
|---|---|---|
| High-Performance Liquid Chromatography (HPLC) | Purity & Related Substances Quantification | Assess area percent purity (often required). Use reversed-phase high-performance liquid chromatography (RP-HPLC) or hydrophilic interaction chromatography (HILIC) mode with a PDA detector to separate and quantify synthetic byproducts and chemical impurities. |
| Mass Spectrometry (MS) | Definitive Identity & Molecular Weight Confirmation | Structural verification. High-resolution accurate mass (HRAM-MS, such as Orbitrap or TOF) is used to verify accurate mass and empirical formula. Tandem mass spectrometry (MS) is essential for confirming fragmentation patterns and distinguishing structural isomers. |
| Nuclear Magnetic Resonance (NMR) Spectroscopy | Unambiguous Chemical Structure Confirmation | Use and to confirm the connectivity of each atom and the presence of unexpected chemical functional groups. This is essential for confirming the designed regio- and stereochemistry. |
| Karl Fischer Titration | Residual Water Content Quantification | Determine water content. This is essential for linkers containing water-sensitive reactive groups (e.g., esters, hydrazones), as excess water can cause premature hydrolysis and reduce conjugation efficiency. |
| Gas Chromatography (GC) | Residual Solvent Analysis | Quantify trace amounts of volatile organic solvents (e.g., trace amounts of volatile organic solvents) remaining from synthesis or purification steps. Ensure compliance with international safety guidelines (ICH Q3C limits). |
| Thermal Analysis (/) | Physicochemical Properties & Batch Consistency | Differential scanning calorimetry (DSC) can determine melting points for identity/purity verification. Thermogravimetric analysis (TGA) measures the non-volatile residue (ash content) and degradation behavior. |
Why Choose Creative Biolabs for Crosslinker Quality Control?
Our commitment to scientific excellence and regulatory compliance makes Creative Biolabs a trusted partner in the bioconjugation field.
✅PhD-level Expertise: Our analytical chemistry and bioconjugation teams are led by experts with decades of experience in small molecule synthesis, characterization, and large molecule conjugation.
✅Advanced Analytical Platforms: We utilize a fully validated suite of modern instrumentation (UHPLC, Orbitrap MS, 600 MHz NMR) to ensure the highest accuracy and sensitivity for impurity detection.
✅Regulatory Focus: All of our quality control protocols and documentation are designed to meet or exceed the requirements of global regulatory agencies, streamlining your new drug application process.
✅Scalability: Our services range from milligram-scale research batches to multi-kilogram production, seamlessly supporting your project from discovery to commercialization.
How to Perform Quality Control on Crosslinkers?
Effective quality control of bioconjugation crosslinkers is a multifaceted approach, focusing on chemical identity, purity, reactivity, and physical properties. The quality control process must verify the following key parameters:
The robust quality control strategy for crosslinking agents is multi-layered, ranging from basic characteristics to highly specific functional analysis. The process of Creative Biolabs includes:
 Specification Setting
Specification Setting
Based on the mechanism of action of the crosslinking agent, strict acceptance criteria are defined for identity, purity, efficacy, and other key quality attributes (CQA).
 Analysis and Testing
Analysis and Testing
A series of orthogonal analysis techniques are used to thoroughly investigate the crosslinking agent.
 Data Analysis and Reporting
Data Analysis and Reporting
Compare the results with predefined specifications and provide a Certificate of Analysis (CoA) for each batch.
 Stability Study
Stability Study
Evaluate the stability of crosslinking agents under various storage conditions (such as temperature and humidity) to determine shelf life and processing procedures.
Frequently Asked Questions
Q: How to choose the appropriate crosslinking agent for my protein conjugation?
A: First, identify the types of functional groups on the protein (such as the primary amine of lysine, the thiol group of cysteine). Then select a crosslinking agent with reactive functional groups that is specifically targeted at these functional groups. In order to control orientation and avoid unnecessary polymerization, bifunctional crosslinking agent can be considered. Finally, consider the protein size when selecting a spacing arm length.
Q: How do I store lyophilized crosslinkers to maintain their stability?
A: If stored properly, freeze-dried crosslinking agents can be stable for 6-12 months, but they may degrade when exposed to moisture, light, or high temperatures. Follow the following storage guidelines:
- Temperature: Store at -20 °C (long-term storage,>6 months) or 4 °C (short-term storage, <1 month). Avoid storing at room temperature, as heat can accelerate chemical degradation (for example, NHS esters decompose 10 times faster at 25 ° C than at -20 °C).
- Moisture proof: Place the crosslinking agent in a dryer containing anhydrous calcium chloride or molecular sieve. When opening the small bottle, work quickly in a dry environment (such as a low humidity fume hood) to prevent moisture absorption. Seal the small bottle tightly with Parafilm after use.
- Photosensitivity: Some crosslinking agents (such as sulfonyl SANPAH and other photosensitive crosslinking agents, FITC labeled crosslinking agents) degrade under light exposure. Store these in amber small bottles or wrap transparent bottles with aluminum foil.
Q: How does crosslinker purity affect the drug-to-antibody ratio (DAR)?
A: Low purity means that a portion of the material you weigh out and use in the reaction is inactive or byproducts. Inactive material reduces the overall conjugation yield. Byproduct impurities can react nonspecifically, resulting in uneven or unstable conjugation. Both of these situations lead to decreased control and increased heterogeneity in the ADC mixture, complicating characterization and affecting the expected DAR.
Q: Why do I need NMR if I have high-resolution MS data?
A: HRAM-MS provides accurate mass and molecular formula, but it cannot distinguish between all structural isomers or definitively confirm the stereochemistry and regiochemistry of the molecule. NMR, especially 2D NMR, provides a unique "fingerprint" of atomic connectivity and chemical environment, providing unambiguous structural confirmation, which is critical for regulatory filings and complex proprietary linkers.
Q: How can I confirm successful cross-linking?
A: Validation depends on the application, but typically involves quantifying coupling efficiency and verifying biological activity. Common methods include:
- SDS-PAGE: For protein or protein payload conjugation (such as antibody HRP), SDS-PAGE separates the cross-linked products by size. Cross linked complexes exhibit higher molecular weight bands than unmodified biomolecules. For example, the antibody HRP conjugate (150 kDa antibody+44 kDa HRP) displays a~194 kDa band, while the unmodified antibody displays a 150 kDa band.
- HPLC or UHPLC: For ADC or fluorophore conjugates, reverse phase HPLC separates coupled and unmodified species based on hydrophobicity. Calculate the conjugation efficiency as (peak area of conjugated product/total peak area) * 100%.
Conclusion
Stringent crosslinker quality control (QC) is an essential step in the successful development of antibody-drug conjugates (ADCs). The crosslinker serves as the critical molecular link between the antibody and the cytotoxic drug, and its identity, purity, and potency directly determine the stability, homogeneity, and therapeutic safety of the final ADC product. Impure crosslinkers can lead to inconsistent drug-to-antibody ratios (DARs) and potentially cause premature drug release, leading to off-target toxicities.
Overview of What Creative Biolabs Can Provide
Creative Biolabs is a global leader in providing high-quality and innovative services for biocoupling and drug discovery. With a profound understanding of the chemical complexity of crosslinking agents, we offer a wide range of services to support your research and development needs. If you are interested in our bioconjugation services, please feel free to contact us for more details.
Recommended products
Reference
- Yammine P, El Safadi A, Kassab R, et al. Types of crosslinkers and their applications in biomaterials and biomembranes. Chemistry, 2025, 7(2): 61. https://doi.org/10.3390/chemistry7020061. Distributed under Open Access license CC BY 4.0, without modification.
