Introduction What We Can Offer? Why Choose Us? FAQs Featured Services Featured Products
Accelerate Your Research and Development!
Chemotherapy-induced neutropenia (CIN) remains a significant challenge in cancer treatment, leading to increased infection risk and treatment delays. Creative Biolabs offers advanced Complement System Therapeutics, providing a professional solution to accelerate your drug discovery process and mitigate CIN complications. Are you currently facing the critical issues of prolonged neutropenia or severe infection risks during chemotherapy? Our advanced therapeutic antibody solutions help you accelerate neutrophil recovery and reduce infection burden through innovative complement modulation strategies, ensuring safer and more effective cancer treatment regimens.
Contact our team to get an inquiry now!
Introduction
Chemotherapy-induced neutropenia (CIN) is a common and severe side effect of cytotoxic chemotherapy, characterized by a significant reduction in circulating neutrophils. This condition renders patients highly susceptible to life-threatening infections, often necessitating dose reductions or delays in chemotherapy, thereby compromising treatment efficacy.
Related Molecular Mechanisms in the Complement System
This defense cascade constitutes an essential component of innate immunity, consisting of serum proteins that, when activated, are vital for combating pathogenic threats and removing immune complexes along with apoptotic cells. However, dysregulation or excessive activation of this system can contribute to tissue damage and disease. In the context of chemotherapy, various cytotoxic agents can inadvertently activate the complement cascade through direct or indirect mechanisms. This activation leads to the generation of potent anaphylatoxins, particularly C3a and C5a, and the formation of the membrane attack complex (MAC).
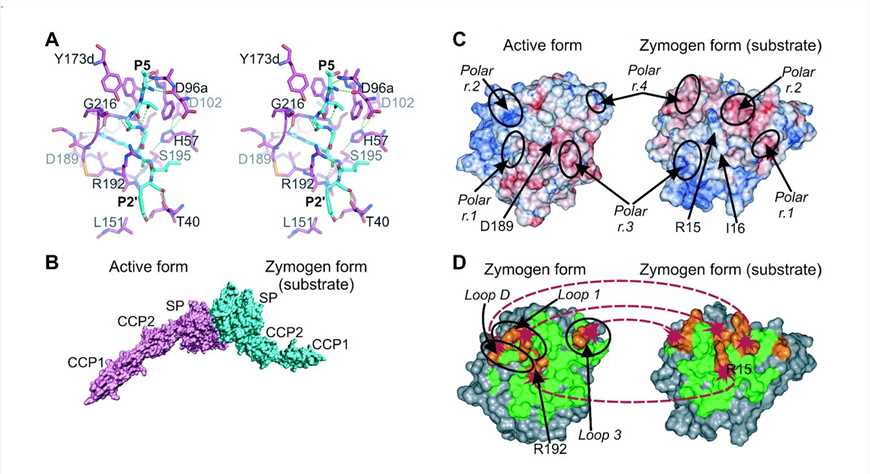
Fig. 1 Schematic diagram of the proposed autoactivation mechanism of MASP-2.1, 2
C5a
Studies indicate that C5a, a powerful chemoattractant and inflammatory mediator, contributes significantly to neutrophil apoptosis and their subsequent clearance. Furthermore, complement activation can directly impair bone marrow function, suppressing granulopoiesis and hindering the replenishment of neutrophil populations. This dual detrimental effect—accelerated neutrophil destruction and impaired production—exacerbates the severity and duration of neutropenia. Understanding these intricate signaling pathways offers a compelling therapeutic avenue: by modulating specific components of the complement system, it is possible to mitigate the adverse effects of chemotherapy on neutrophil counts and improve patient outcomes in CIN.
MASP-2
MASP-2, an enzyme encoded by the MASP2 gene, is a vital component of the complement system, particularly the lectin pathway. Sharing similarities with C1s, MASP-2 activates upon MBL binding to pathogen mannose residues, subsequently cleaving complement components C4 and C2. MASP-2 is essential for the complement-activated lectin pathway. Its deficiency, common due to genetic polymorphisms, can impact innate immunity and individual susceptibility to infection. Critically, research indicates that oncology patients lacking MASP-2 commonly exhibit heightened frequency or severity of febrile episodes and chemotherapy-induced neutropenia (FN).
What We Can Offer?
Creative Biolabs offers products and services for complement system therapeutics in chemotherapy-induced neutropenia:
-
Recombinant Complement Proteins: High-purity recombinant human and animal complement proteins for research.
-
Complement Inhibitors & Modulators: Diverse small molecules and biologics targeting complement components (e.g., C3, C5, C5aR) for screening.
-
Therapeutic Antibodies: Development of highly specific antibodies against key CIN-implicated complement factors, including anti-C5a.
-
ELISA Kits: Ready-to-use ELISA kits for quantifying complement activation products (e.g., C3a, C5a, sC5b-9) and biomarkers.
-
Complement Activation Assays: Comprehensive in vitro assays for complement pathway activation (classical, alternative, lectin) in response to stimuli.
Why Choose Us?
Creative Biolabs leads in complement system therapeutics, offering unmatched expertise and innovative solutions for chemotherapy-induced neutropenia. Our commitment to scientific rigor and client success makes us your ideal partner.
Key Advantages:
-
Deep Scientific Expertise: Our team of seasoned biologists and immunologists ensures a profound understanding of CIN mechanisms and therapeutic targets.
-
Comprehensive Service Portfolio: We offer end-to-end solutions, from target identification to preclinical evaluation, providing a seamless project workflow.
-
Cutting-Edge Technology Platforms: Utilizing state-of-the-art platforms for high-throughput screening, advanced protein engineering, and robust in vitro and in vivo models, we accelerate effective complement modulator development.
-
Proven Track Record: Our success is evidenced by numerous collaborations and promising therapeutic candidates. (Published Data available upon request).
-
Customized Collaborative Approach: We tailor services to specific project requirements, fostering a truly collaborative research environment.
-
Rigorous Quality Control: Our process adheres to stringent quality standards, ensuring reliable data and high-quality deliverables.
Secure the Creative Biolabs Benefit - Solicit Pricing Now
FAQs
Q: How do complement-targeting therapies specifically address the reduction of white blood cells after chemotherapy?
A: Targeting the complement system helps by preventing the excessive activation of complement proteins, such as C5a, which are known to contribute to the destruction of white blood cells, particularly neutrophils, and can also impair bone marrow function. By inhibiting these specific pathways, the therapy aims to reduce neutrophil loss and support their recovery, thereby mitigating the severity and duration of the low white blood cell count.
Q: What are the key advantages of modulating the complement system for this condition compared to traditional supportive care?
A: Modulating the complement system offers a more targeted approach by addressing a root cause of neutrophil depletion, rather than just managing its consequences. This can lead to faster and more robust neutrophil recovery, potentially reducing the need for broad-spectrum antibiotics and minimizing the risk of severe infections. It also allows for more consistent chemotherapy dosing, which can improve overall treatment effectiveness.
Q: Are there any specific considerations or precautions when developing or applying these therapies?
A: Developing these therapies requires careful consideration of specificity to avoid off-target effects on the beneficial aspects of the immune system. Precise targeting of the pathological complement activation while preserving its protective functions is crucial. In application, patient selection and monitoring for potential immune-related side effects are important to ensure optimal safety and efficacy.
Q: How can one assess the efficacy of a complement-based therapeutic candidate in preclinical studies?
A: Effectiveness can be determined through integrated in vitro and in vivo methodologies. In vitro studies might involve evaluating the candidate's ability to inhibit complement activation in response to chemotherapy agents or to protect neutrophils from damage in cell culture. In vivo studies typically involve animal models of chemotherapy-induced neutropenia, where neutrophil counts, infection rates, and bone marrow recovery are monitored after treatment with the therapeutic candidate.
Q: What are the future prospects for complement-based therapies in managing this condition?
A: The future prospects are promising, with ongoing research exploring new targets within the complement cascade and developing more refined therapeutic agents. As our understanding of the complement system's role in chemotherapy-induced side effects deepens, these therapies could become a cornerstone of supportive care, potentially leading to personalized medicine approaches that optimize patient outcomes and reduce treatment-related complications.
Featured Services
Feature Products
Reference
-
Gál, Péter, et al. "A true autoactivating enzyme: structural insight into mannose-binding lectin-associated serine protease-2 activations." Journal of Biological Chemistry 280.39 (2005): 33435-33444. DOI: 10.1074/jbc.M506051200. Distributed under an Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections: