Introduction of Complement Factor P
Complement Factor P (CFP), also known as properdin, is the only known positively charged (pI>9.5) regulator of complement system. Factor P is a 53-kDa monomer composed of 6 globular domains that are homologous to the thrombospondin type 1 repeat (TSR), labeled TSR 1-6. Consisted of 442 amino acid residues, factor P is 26 nm in length and 2.5 nm in diameter, meanwhile, each subunit harbors a single N-glycosylation site in TSR-6 and is C-mannosylated at 14 different tryptophans, making properdin one of the most highly mannosylated proteins known. Significantly, native factor P subunits form head-to-tail dimers, trimers, and tetramers that resemble rods, triangles, and squares, respectively. And different formations usually present the fixed ratio 22:52:28.
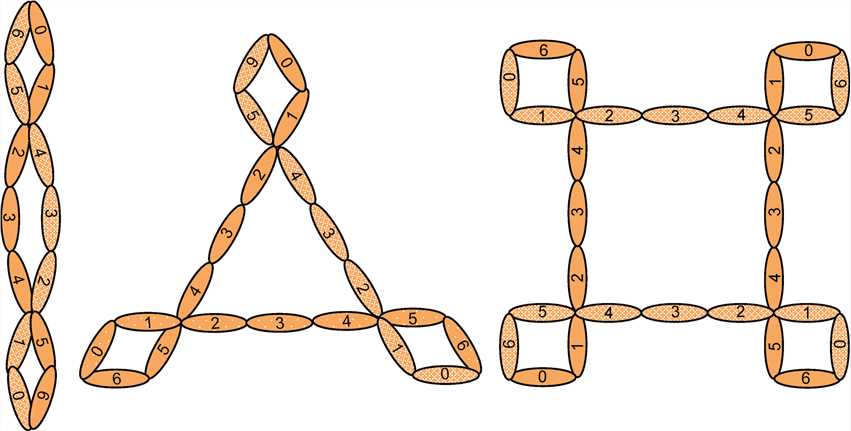
Fig.1 Factor P structure.1
Function of Complement Factor P
Factor P is historically recognized as a stabilizing component of the alternative pathway convertases, the central enzymes of the complement cascade. However, weighty evidence has revealed that factor P can also bind to target cells, phagocyte receptors, and serum regulators, providing a platform for convertase assembly and function, and promotes target phagocytosis.
-
Direct Regulation of Alternative Pathway Activity
Factor P can enhance alternative pathway activity via two ways: (1) acting as a positive regulator of pre-existing alternative pathway activity or (2) initiating new alternative pathway activity. Acting as a positive regulator, factor P can stabilize the C3 convertase (C3bBb) of the alternative pathway, increasing their activity five to ten-fold. Factor P acts as a pattern recognition molecule to bind selectively to specific surfaces upon which it recruits C3b or C3(H2O) to direct and trigger alternative pathway.
-
Binding to Apoptotic T Cells to Promote Phagocytosis
Factor P can bind early apoptotic T cells, which promotes their phagocytic uptake by macrophages and dendritic cells. Additionally, surface-bound factor P directed deposition of C3b activation fragments on apoptotic T cells to enhance complement activation.
-
Binding to Microbial Pathogens
Factor P is showed directly to bind Neisseria gonorrhoeae and the resulting bacteria:properdin complexes induce C3 deposition to the microbial surface, which is beneficial for activation of complement pathway. These observations may account for why properdin-deficient individuals are particularly sensitive to meningococcal disease.
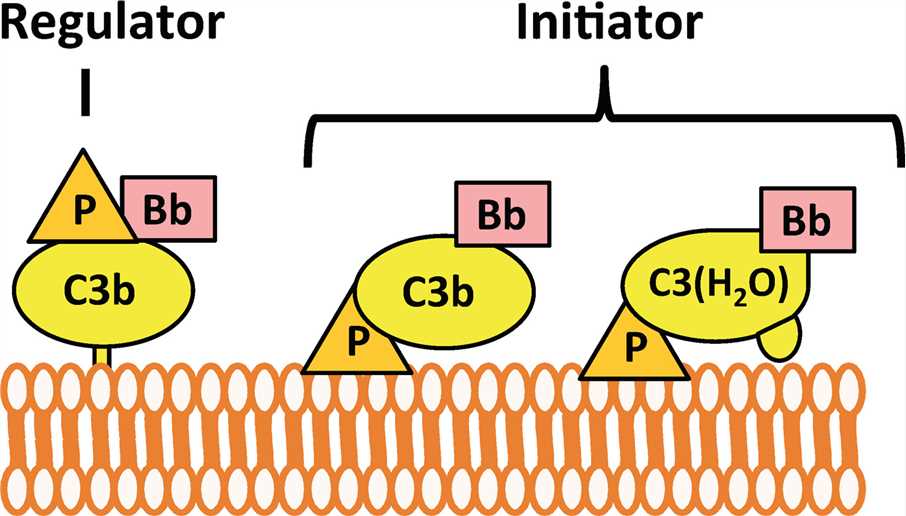
Fig.2 Function of factor P.1
Factor P and Disease
Factor P has been invariably found to be critical for alternative pathway activation, so a number of disorders mediated by the alternative pathway have close relevance with factor P. Indeed, apart from N. meningitides infection in P-deficient individuals, some autoimmune disease, most notably the development of systemic lupus erythematosus (SLE) and renal diseases are also associated with factor P. So factor P may represent a promising therapeutic target in clinical.
With rich research experiences in drug discovery and complement therapeutic field, Creative Biolabs' outstanding research team are providing high-quality biotherapeutics development services based on the complement system. We offer turn-key or ala carte services customized to our client’s needs. If you are interested in our platform, please contact us for detailed information.
Published Data
Innate lymphoid cells (ILCs), crucial for immune defense against pathogens and stressed cells like tumors, utilize various surface receptors to identify microbial and other non-microbial signals. Researchers examined NKp46, a receptor preserved across mammals, found on mature NK cells and subsets of ILC1 and ILC3. CFP, an enhancer of the alternative complement pathway, interacts with NKp46 on NK cells and certain ILC subsets. In murine models, CFP's protective action was reliant on NKp46 and group 1 ILCs, highlighting a cooperative interaction between ILCs and the alternative complement pathway in combating bacterial infections, CFP can be a potential therapeutic target.
Reference
-
Blatt, Adam Z., Sabina Pathan, and Viviana P. Ferreira. "Properdin: a tightly regulated critical inflammatory modulator." Immunological reviews 274.1 (2016): 172-190. Distributed under Open Access license CC BY 4.0, without modification.
Related Product
For Research Use Only.
Related Sections:


