Complement C1QC
Complement C1, the first member of the classical complement pathway, is a protein complex composed of the subcomponents C1q, C1r, and C1s. Among these subcomponents, C1q is a 460 kDa protein made up of 18 polypeptide chains, 6 C1QA, 6 C1QB, and 6 C1QC chains, respectively. Human complement C1q subcomponent subunit C (C1QC) is a 24 kDa polypeptide chain with 245 amino acids forming a lollipop-like structure. In the lollipop-like structure of C1QC, about 81 amino acids near the N terminus yield the collagen-like ‘rod’, and ~136 residues at the C terminal form the globular ‘head’ structure. The collagen-like region of C1QA, C1QB, and C1QC link to each other forming a triple-helical structural strand. And then C1QC of one strand is linked to the adjacent C chain by a disulfide bond forming an ABC-CBA doublet. 6 such triple helical strands or 3 ABC-CBA doublets non-covalently combine resulting in the intact complement C1q with a bouquet-like structure.
The human C1QA, C1QB, and C1QC encoded genes are arranged in the order A-C-B on chromosome 1. Abnormal expression of any C1QA, C1QB, and C1QC will lead to defective C1q function, causing the C1q to fail to bind to antigen-antibody complex and activate the classical complement pathway. Deficiency of C1QC or C1q has been associated with immunodeficiency and autoimmune diseases.
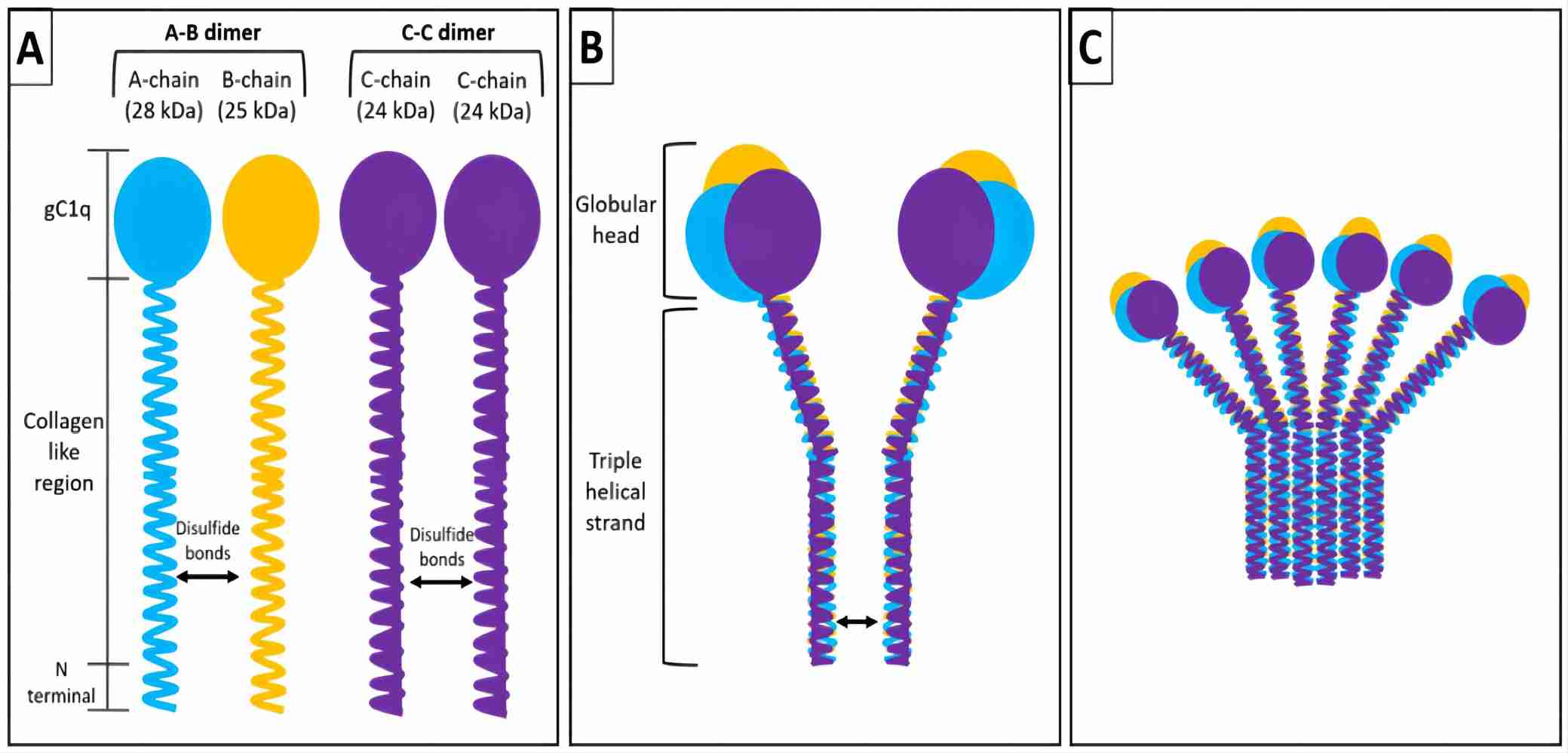 Fig. 1 Structure and assembly of C1QC subunit.1
Fig. 1 Structure and assembly of C1QC subunit.1
Reference
-
Ain, Danyaal, et al. "The role of complement in the tumor microenvironment." Faculty Reviews 10 (2021).
For Research Use Only.

 Fig. 1 Structure and assembly of C1QC subunit.1
Fig. 1 Structure and assembly of C1QC subunit.1