Complement C4BPA
Complement C4 binding protein alpha chain (C4BPA) is an important component of the C4BP complex. Usually, human C4BPA is composed of six/seven identical α chains, each α chain consists of 8 complement control protein (CCP) repeat domains. By comparison, mouse C4BPA consists of 7 α chains and each α chain contains 6 CCP domains. There are two cysteine residues and an amphipathic a-helix at the C-terminal of each α chain, which is responsible for intracellular polymerization of the C4BP complex. On the α chain lie different binding sites of various ligands.
Human C4BPA is a 67 kDa protein with 597 amino acids. The CCP1-3 domain of the α chain is the binding site of activated complement C4b. When the C4BPA binds to the cell-bound C4d fragment, the C4b will be cleaved by the complement factor I, a serine protease that regulates complement activation. Thus, C4BPA serves as a cofactor of factor I or a negative regulator, inactivating the C4b into C4c and C4d, thereby inhibiting the over activation of the complement classical pathway and complement lectin pathway. The abnormality of C4BPA is likely to cause the over-activation or dysfunction of the complement system, causing disorders like acute poststreptococcal glomerulonephritis and protein S deficiency.
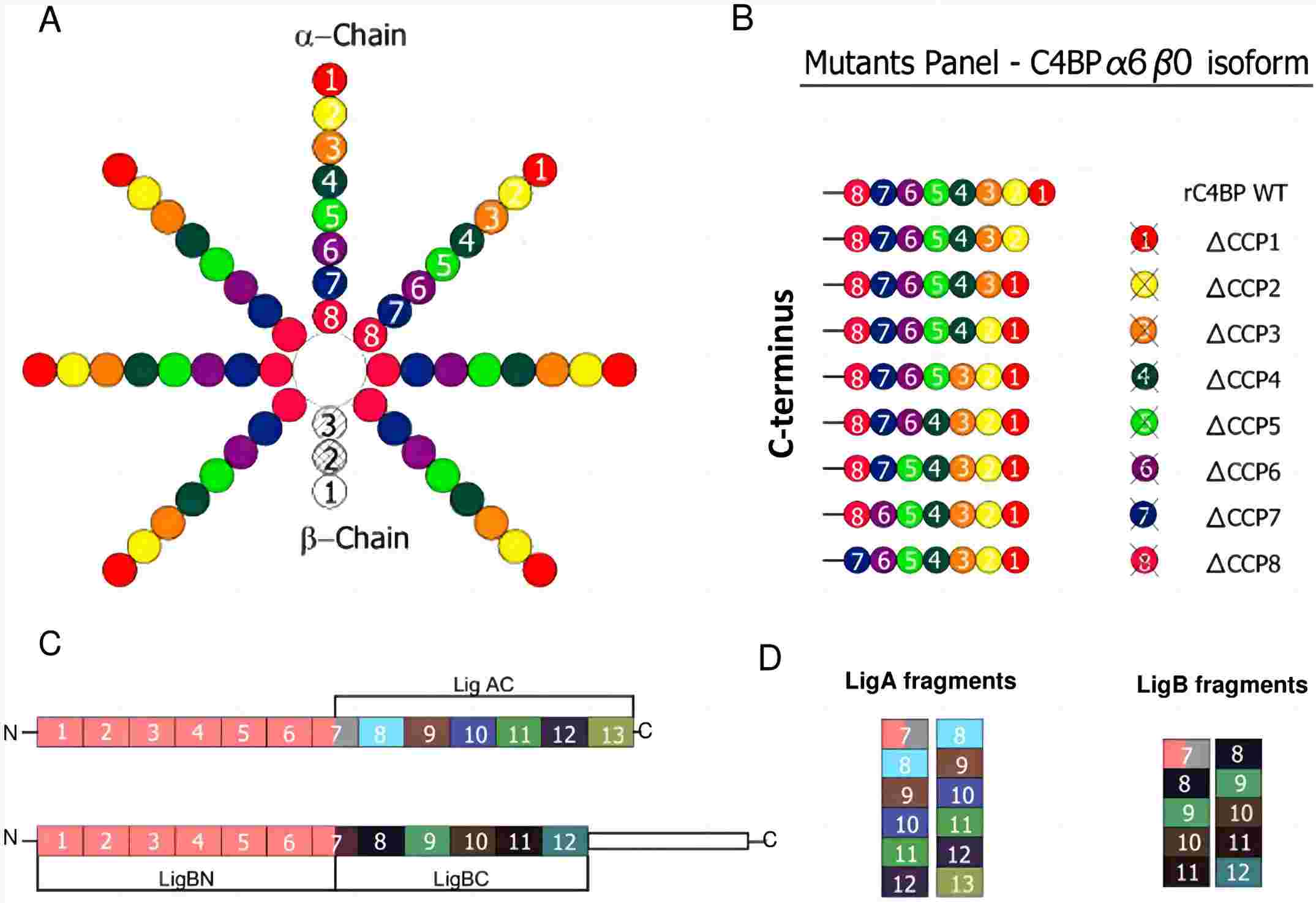 Fig. 1 The structure of C4BP.1
Fig. 1 The structure of C4BP.1
Reference
-
Breda, Leandro CD, et al. "Fine mapping of the interaction between C4b-binding protein and outer membrane proteins LigA and LigB of pathogenic Leptospira interrogans." PLoS neglected tropical diseases 9.10 (2015): e0004192.
For Research Use Only.

 Fig. 1 The structure of C4BP.1
Fig. 1 The structure of C4BP.1