Product List Background C8A Functional Service
Background
Complement component 8 (C8) plays an essential role in the assembly of membrane attack complex (MAC), the effector of the complement system that can lyse the target cells. As a functional complement protein, C8 is a heterotrimer protein composed of three subunits, α chain, β chain, and γ chain, in which complement C8A (C8A) is the α chain of C8. Human C8A is a 584-amino acid 65 kDa polypeptide, composed of a MAC perforin domain and other four modules (two thrombospondin modules, an LDL-related module, and an epidermal growth factor module). The MAC perforin domain of C8A has a characterized L-shaped fold that is similar to bacterial cholesterol-dependent cytolysins, which contains two regions (transmembrane b hairpin 1 (TMH1) and TMH2) responsible for insert into the target membrane. And the N-terminal of C8A lies a binding site for the γ chain that covalently links to C8G through a disulfide bond.
Mutations in the C8A gene cause complement C8 deficiency type I, a rare autosomal recessive inheritance disease, leading to increased susceptibility to recurrent bacterial infections, especially neisserial infections. C8A deficiency also closely associates with immunodeficiency due to a late component of complement deficiency.
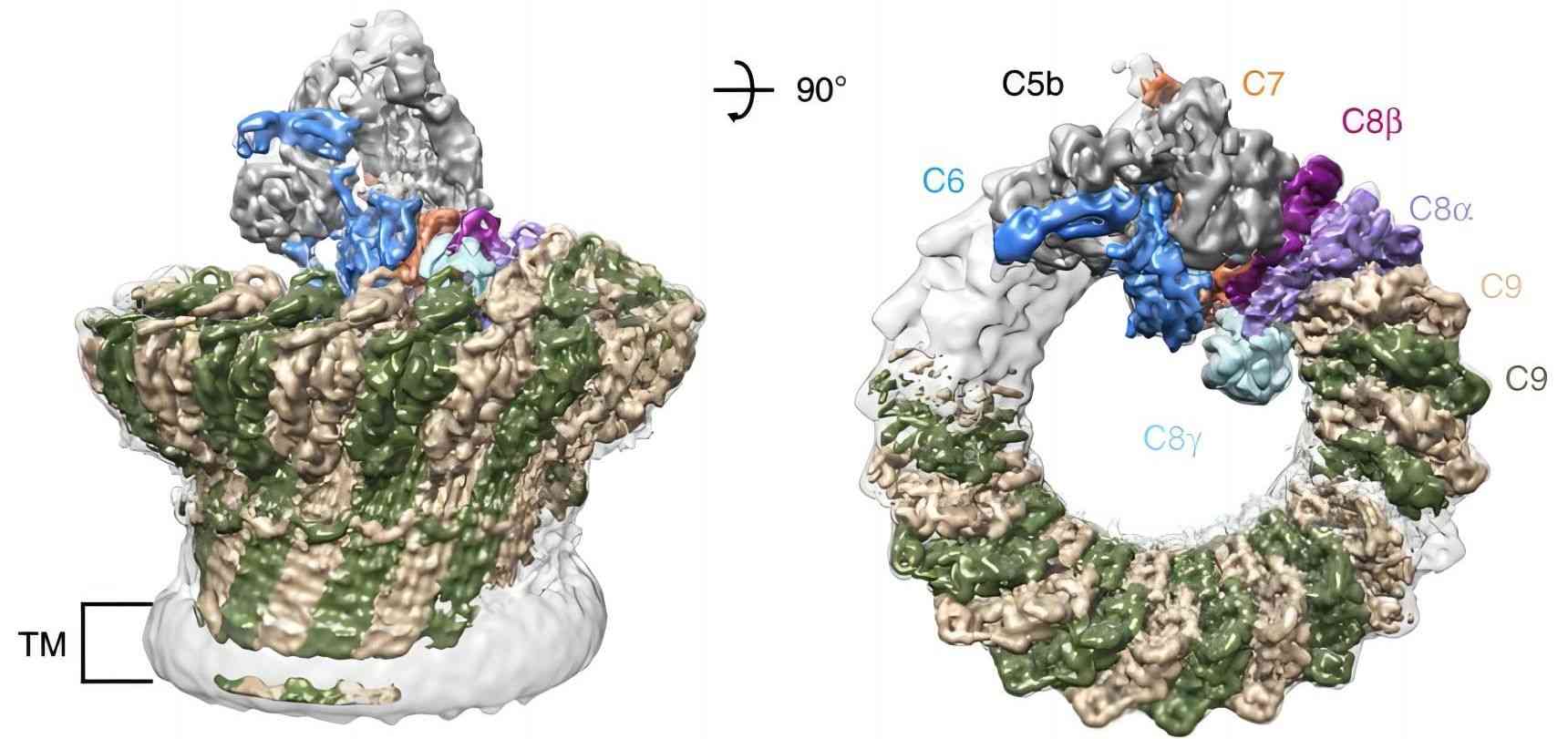 Fig.1 Structural role of C8A in the formation of the MAC pore.1
Fig.1 Structural role of C8A in the formation of the MAC pore.1
C8A Functional Service
Creative Biolabs offers a comprehensive array of products linked to C8A, encompassing ELISA kits for C8A detection, recombinant C8A proteins, C8A protein lysates, and reporter vectors containing the C8A gene. These precision-engineered reagents are crucial for progressing research aimed at creating therapeutic approaches for diverse diseases.
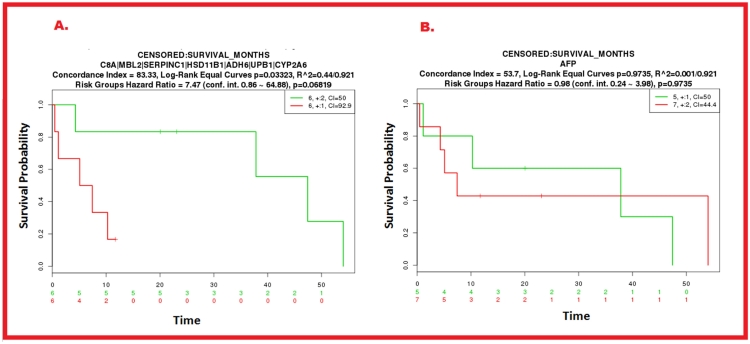 Fig.2 Kaplan-Meier curve comparison between the traditional HCC biomarker AFP and seven potential circulating biomarkers (including C8A).2
Fig.2 Kaplan-Meier curve comparison between the traditional HCC biomarker AFP and seven potential circulating biomarkers (including C8A).2
Hepatocellular carcinoma (HCC) ranks former in most prevalent cancer globally. The specificity and sensitivity of current circulating biomarkers are inadequate for broad clinical utility. Researchers developed an objective method to refine the analysis of extensive datasets from cutting-edge technologies, utilizing seven gene and protein databases to discover 731 liver-specific proteins. This approach incorporated immunohistochemistry, proteome databases, and bioinformatics tools. Through interaction analysis with midkine (MDK), dickkopf-1 (DKK-1), and alpha-fetoprotein (AFP), along with HCC-specific elements, seven new potential biomarkers, including complement component C8A, were identified. This integrative methodology facilitates efficient biomarker prioritization, reducing experimental costs and speeding up validation for other diseases.
Creative Biolabs provides a diverse range of tailored C8A-related services, encompassing detailed interaction analyses and other functional capabilities. These meticulously developed offerings are designed to support clients in advancing their notable scientific research and clinical pursuits.
References
-
Menny, Anaïs, et al. "CryoEM reveals how the complement membrane attack complex ruptures lipid bilayers." Nature communications 9.1 (2018): 5316. Distributed under Open Access license CC BY 4.0, without modification.
-
Awan, Faryal Mehwish, et al. "Identification of circulating biomarker candidates for hepatocellular carcinoma (HCC): an integrated prioritization approach." PloS one 10.9 (2015): e0138913.


 Datasheet
Datasheet Fig.1 Structural role of C8A in the formation of the MAC pore.1
Fig.1 Structural role of C8A in the formation of the MAC pore.1
 Fig.2 Kaplan-Meier curve comparison between the traditional HCC biomarker AFP and seven potential circulating biomarkers (including C8A).2
Fig.2 Kaplan-Meier curve comparison between the traditional HCC biomarker AFP and seven potential circulating biomarkers (including C8A).2
