Product List Background CD55 Functional Service
Background
Complement decay-accelerating factor, also known as CD55 or DAF, is 70 kDa glycoprotein that is encoded by the CD55 gene. The encoded protein is found on the membranes of vascular endothelial cells, peripheral blood cells, placenta, and many types of epithelial cells. DAF protein contains four complement control protein (CCP) domains with a single N-linked glycosylation site between CCP1 and CCP2, a heavily O-glycosylated membrane proximal domain, and a glycosylphosphatidylinositol (GPI) anchor which anchors the protein to phospholipids on the cell membrane. Without the anchor phospholipid, DAF can also exist in a soluble form and has been found in many body fluids including plasma, urine, tears, saliva, synovial and cerebrospinal fluids.
DAF plays a crucial role in protecting host cells from autologous complement attacks. It prevents the formation of C4b2a and C3bBb through binding to cell-associated C4b and C3b polypeptides, thereby suppressing amplification convertases of the complement cascade. It also inhibits complement activation by destabilizing and preventing the formation of C3 and C5 convertases. Moreover, DAF is a receptor for some viruses such as several echoviruses and certain types of echo- and coxsackie B-viruses. Some pathogens such as HIV have been found to transfer GPI-anchored DAF into their cell membranes in a functionally active form.
DAF deficiencies are associated with several diseases including paroxysmal nocturnal haemoglobinuria, and autoimmune diseases. Recent studies reported DAF is involved in tumorigenesis and the DAF expression levels are increased in many types of malignant tumors.
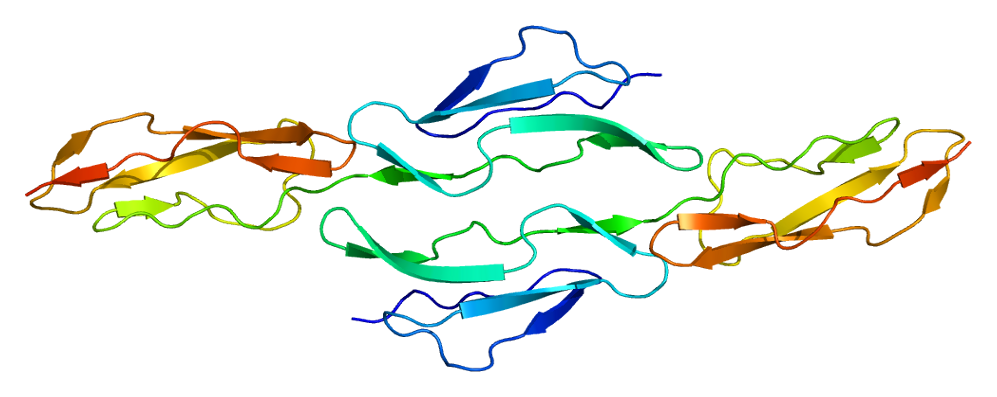 Fig.1 Structure of CD55. Distributed under CC BY-SA 3.0, from Wiki, without modification.
Fig.1 Structure of CD55. Distributed under CC BY-SA 3.0, from Wiki, without modification.
CD55 Functional Service
Creative Biolabs provides a comprehensive range of CD55-related products, encompassing anti-CD55 Antibodies, CD55 ELISA Assay kits, and CD55 Proteins. These products are designed to investigate the role of CD55 in protecting cells from complement-mediated lysis and its involvement in autoimmune diseases and cancer immunology.
CD55 regulates the activity of the complement system by accelerating the decay of the classical and alternative C3/C5 convertase, preventing complement-mediated damage to host cells. Functional tests for CD55 assess its ability to protect cells from complement attack and its interaction with complement proteins. Various methods, such as flow cytometry, ELISA, and complement hemolysis assays, are used to evaluate CD55 activity.
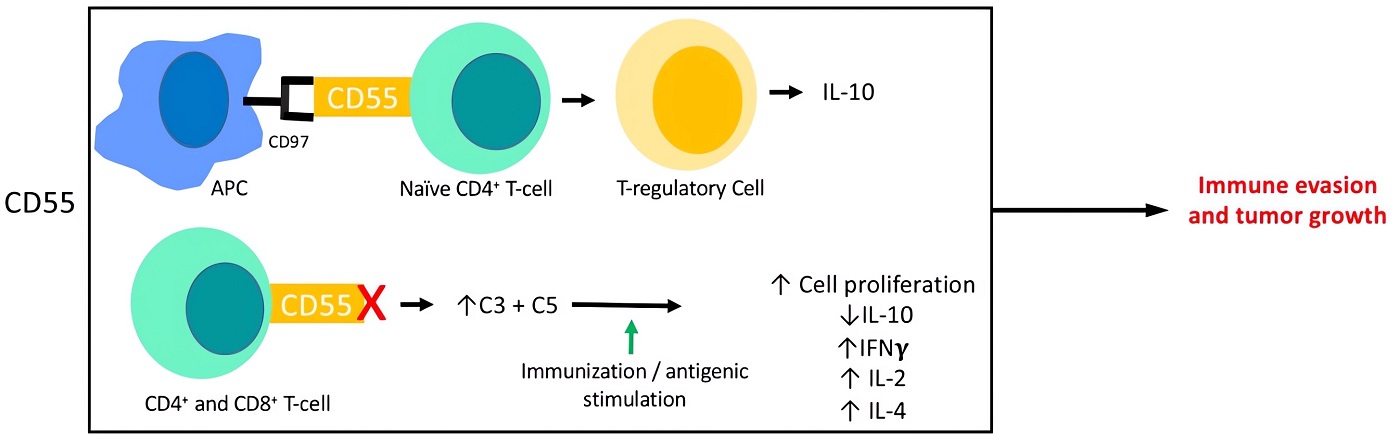 Fig.2 The interaction of CD55 with the adaptive immune response.1
Fig.2 The interaction of CD55 with the adaptive immune response.1
Our CD55-related products can be utilized in conjunction with these techniques to facilitate accurate and reliable functional analysis. For example, a study using CD55 blocking peptides has demonstrated increased complement activation and cell lysis, showing CD55’s protective role in immune regulation. The examination of CD55 expression in PNH and other autoimmune disease patients by flow cytometry in a study has found that CD55 expression is significantly reduced. Functional tests also help explore CD55’s involvement in tumor immune evasion, as cancer cells often overexpress CD55 to escape complement-mediated destruction.
Creative Biolabs provides CD55-functional services, including customized assays, complement regulation studies, and immune response assessments, to help customers accelerate their research and achieve their scientific goals. Our experienced scientists and state-of-the-art facilities enable us to deliver high-quality, tailored solutions to help clients advance their research.
Reference
-
Geller, Anne, and Jun Yan. "The role of membrane bound complement regulatory proteins in tumor development and cancer immunotherapy." Frontiers in immunology 10 (2019): 1074. Distributed under Open Access license CC BY 4.0. The image was modified by extracting and using only part of the original image.


 Datasheet
Datasheet Fig.1 Structure of CD55.
Fig.1 Structure of CD55.  Fig.2 The interaction of CD55 with the adaptive immune response.1
Fig.2 The interaction of CD55 with the adaptive immune response.1
