Product List Background Factor I Functional Service
Background
Complement factor I (CFI), also known as C3b/C4b inactivator, is a protein that is encoded by the CFI gene. It was firstly identified in 1966 in guinea pig serum and was considered as an enzyme associated with the physiological degradation of the major plasma complement protein C3. Later, it was also indicated to function in the physiological breakdown of the human complement protein C4. By the early 1980s, CFI was revealed to act in the cleavage of the complement protein fragment C3b to form iC3b, and the cleavage of C4b to form C4c plus C4d. The CFI plays an important role in complement activation by cleaving cell-bound or fluid phase C3b and C4b.
CFI is produced primarily in the liver, monocytes, fibroblasts, keratinocytes, and endothelial cells. The factor I encoded by the CFI gene is a 66 kDa polypeptide chain with N-linked glycans at 6 positions. After that, the protein cleaved by furin to mature factor I protein which is a heterodimer consisting of a disulfide-linked heavy chain (residues 19-335, 51 kDa) and light chain (residues 340-583, 37 kDa). The factor I heavy chain acts as an inhibitor in maintaining the enzyme inactivation until it encounters the complex formed by the substrate (C3b or C4b) and a cofactor protein such as factor H and complement receptor. The factor I light chain contains only the serine protease domain which is responsible for specific cleavage of C3b and C4b.
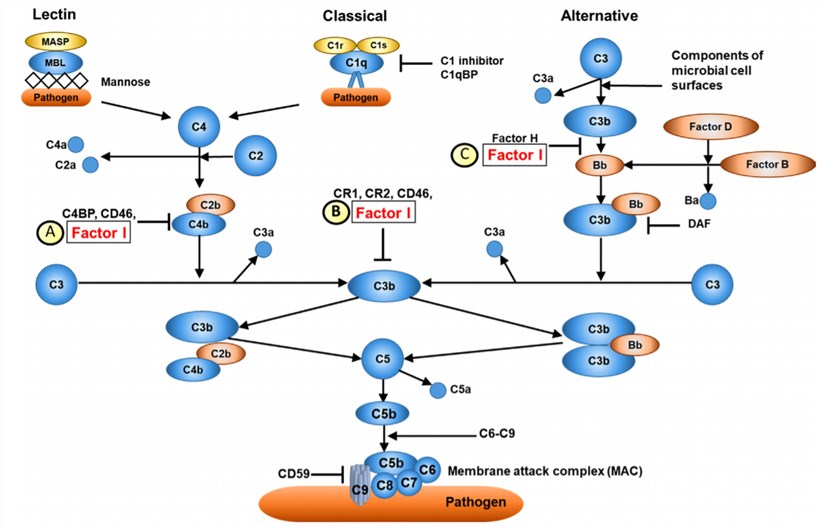 Fig.1 Overview of CFI function.1,3
Fig.1 Overview of CFI function.1,3
Factor I Functional Service
Creative Biolabs provides a wide range of Factor I-related products, encompassing anti-Factor I monoclonal/polyclonal Antibodies, complement Factor I ELISA Assay kits, and complement Factor I Proteins. These products offer high specificity and sensitivity and are designed to support advanced research in immunology, complement system function, and autoimmune diseases.
Complement Factor I is a key regulator of the complement system, and degrades C3b in conjunction with cofactor proteins, which inhibits further complement activation. Impaired Factor I function leads to uncontrolled complement activation, causing diseases like atypical hemolytic uremic syndrome and age-related macular degeneration. Complement Factor I functional testing is used to evaluate its activity and functionality in the blood, typically by measuring Factor I's ability to cleave and inactivate complement components, such as C3b and C4b.
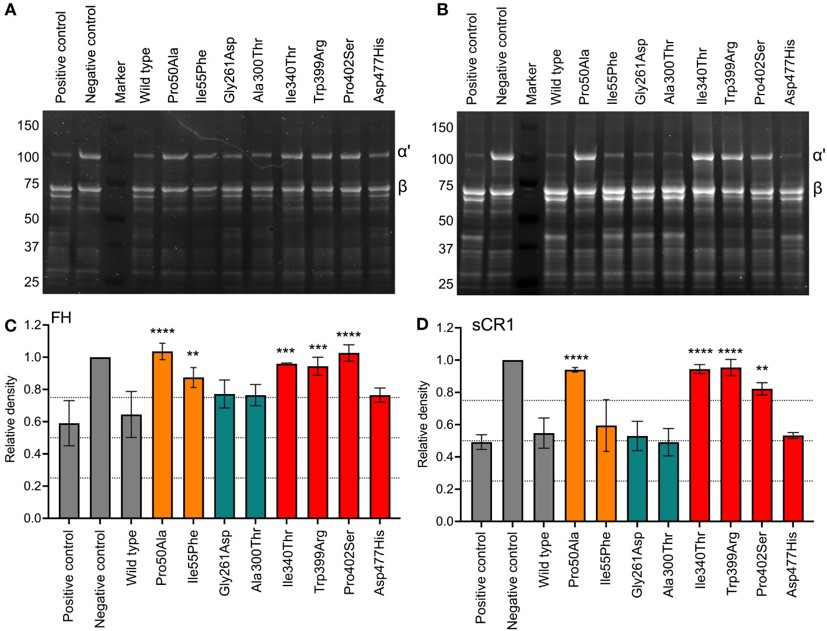 Fig.2 Functional analysis of recombinant Factor I proteins with SDS-PAGE.2,3
Fig.2 Functional analysis of recombinant Factor I proteins with SDS-PAGE.2,3
Our Factor I functional testing services utilize advanced techniques, including enzyme activity assays, ELISA-based assays, and CH50 assay. By employing our high-quality products, researchers can accurately measure Factor I levels, monitor its activity and investigate its involvement in diseases. In a study, functional evaluation of Factor I variants was performed: SDS-PAGE to assess C3b degradation and ELISA to measure the generation of iC3b in the fluid phase demonstrated a significant reduction in enzymatic activity, providing new insights into the available diagnostic tools for evaluating the impact of Factor I mutations.
Creative Biolabs provides comprehensive Factor I-functional services, including custom assay development, functional testing, and data analysis, tailored to meet your research needs and enhance your study outcomes.
References
-
Nanthapisal, Sira, et al. "Cutaneous vasculitis and recurrent infection caused by deficiency in complement factor I." Frontiers in Immunology 9 (2018): 735.
-
Gerogianni, Alexandra, et al. "Functional evaluation of complement factor I variants by immunoassays and SDS-PAGE." Frontiers in Immunology 14 (2023): 1279612.
-
Distributed under Open Access license CC BY 4.0 , without modification.


 Datasheet
Datasheet Fig.1 Overview of CFI function.1,3
Fig.1 Overview of CFI function.1,3
 Fig.2 Functional analysis of recombinant Factor I proteins with SDS-PAGE.2,3
Fig.2 Functional analysis of recombinant Factor I proteins with SDS-PAGE.2,3
