Ficolin-3 (H-ficolin)
Ficolin-3, also named H-ficolin, is a plasma protein first discovered in 1990 as an autoantigen. It is a soluble pattern recognition molecule (PRM) of the innate immune system and binds to acetylated molecules, for example, as found in acetylated carbohydrate structures or on proteins via its fibrinogen-like recognition domain. The fitting patterns recognized by ficolin-3 may be present on apoptotic and necrotic cells, and this offers ficolin-3 the possibility of acting as an initiator of scavenging actions. Besides, ficolin-3 plays an important role in the complement system which can activate the lectin pathway (LP), causing both anti-microbial defense and homeostatic balance. To initiate this activation, ficolin-3 relies on the activation of attached enzymes, i.e., so-called Mannose-binding lectin (MBL)-associated serine proteases (MASPs). When compared to ficolin-1, ficolin-2, MBL, and collectin-LK, ficolin-3 is the most abundant of the five PRMs of the LP.
Research showed that a frameshift mutation in exon 5 (FCN3 + 1637delC) of the FCN3 gene, resulting in premature termination of transcription was demonstrated to be involved in lower levels of ficolin-3 in the heterozygous state. It also revealed that ficolin-3 participate in the clearance of dying host cells. Furthermore, ficolin-3 deficiency is a rare condition and is related to both infection and autoimmune disease including systemic lupus erythematosus (SLE). A study has proven that ficolin-3 deficiency causes an 8-time increased odds of having a disease (p<0.05).
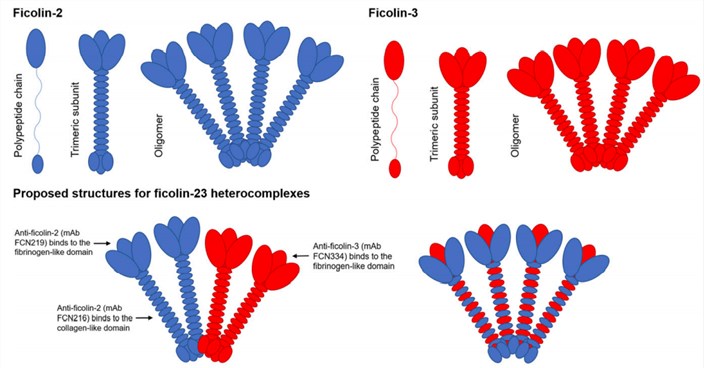 Fig.1 Proposed structures of ficolin-23. (Jarlhelt, 2020)
Fig.1 Proposed structures of ficolin-23. (Jarlhelt, 2020)
Reference
-
Jarlhelt, I., et al. Circulating ficolin-2 and ficolin-3 form heterocomplexes. The Journal of Immunology. 2020,204(7), 1919-1928.
For Research Use Only.

 Fig.1 Proposed structures of ficolin-23. (Jarlhelt, 2020)
Fig.1 Proposed structures of ficolin-23. (Jarlhelt, 2020)