Product List Background MASP1 Functional Service
Background
The complement lectin pathway plays an important role in the clearance of pathogenic microbes. Its activation is mediated by the binding of recognition molecules to glycosylated structures on the surface of a variety of microorganisms, which cannot be achieved without the participation of mannose-associated serine proteases (MASP).
3 MASPs have been identified currently, MASP-1, MASP-2, and MASP-3. MASP-1, also known as mannan-binding lectin serine protease 1, is synthesized as a zymogen with a single-chain polypeptide of 699 residues. The structure of MASP-1 is analogous to C1r and C1s, consisting of 6 domains: a serine protease domain, two complement control proteins (CCPs), and an epidermal growth factor (EGF)-like domain locating in the middle of two CUB (C1r/C1s, urchin-EGF, and bone morphogenetic protein-1) domains. The N-terminal CUB domain and EGF domain are antiparallel, forming a mature MASP homodimer in a Ca2+-dependent manner. The two CUB domains are responsible for binding to the collagen-like domains of recognition molecules, and the serine protease domain has a substrate-binding groove allowing cleavage substrates.
Normally, the recognition molecules are complexed with MASPs. Once recognition molecules bind to pathogen carbohydrate, MASP-1 will undergo a conformation change to be activated by cleavage between the second CCP and serine protease domain. The cleavage generates two active polypeptides, A-chain and B-chain, linked by a disulfide bond. The active MASP-1 then activates MASP-2, which subsequently cleaves complement C2 and C4, leading to the C3 convertase formation and complement activation. Additionally, MASP-1 also is capable of cleaving coagulation factors, such as fibrinogen and factor XIII, involving in coagulation.
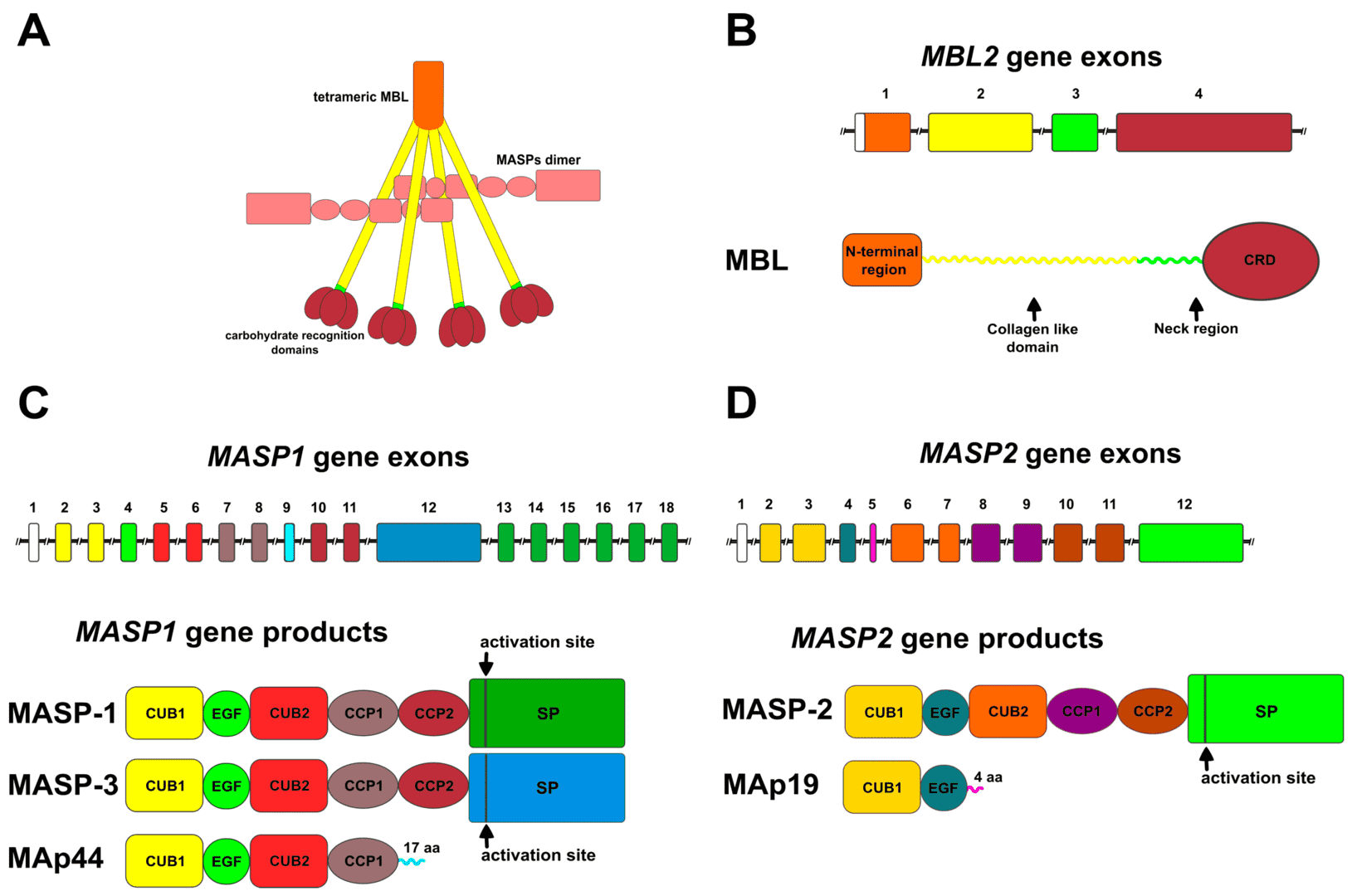 Fig. 1 Structure of MASP1.1, 3
Fig. 1 Structure of MASP1.1, 3
MASP1 Functional Service
Creative Biolabs provides an all-encompassing collection of MASP1-related products, including anti-MASP1 antibodies, ELISA assay kits, human complement MASP1 proteins, and MASP1 blocking peptides. These resources are expertly designed to detect and monitor interactions between antibody domains and the human MASP1 protein, thereby serving a vital function in research focused on therapeutic strategies for diseases.
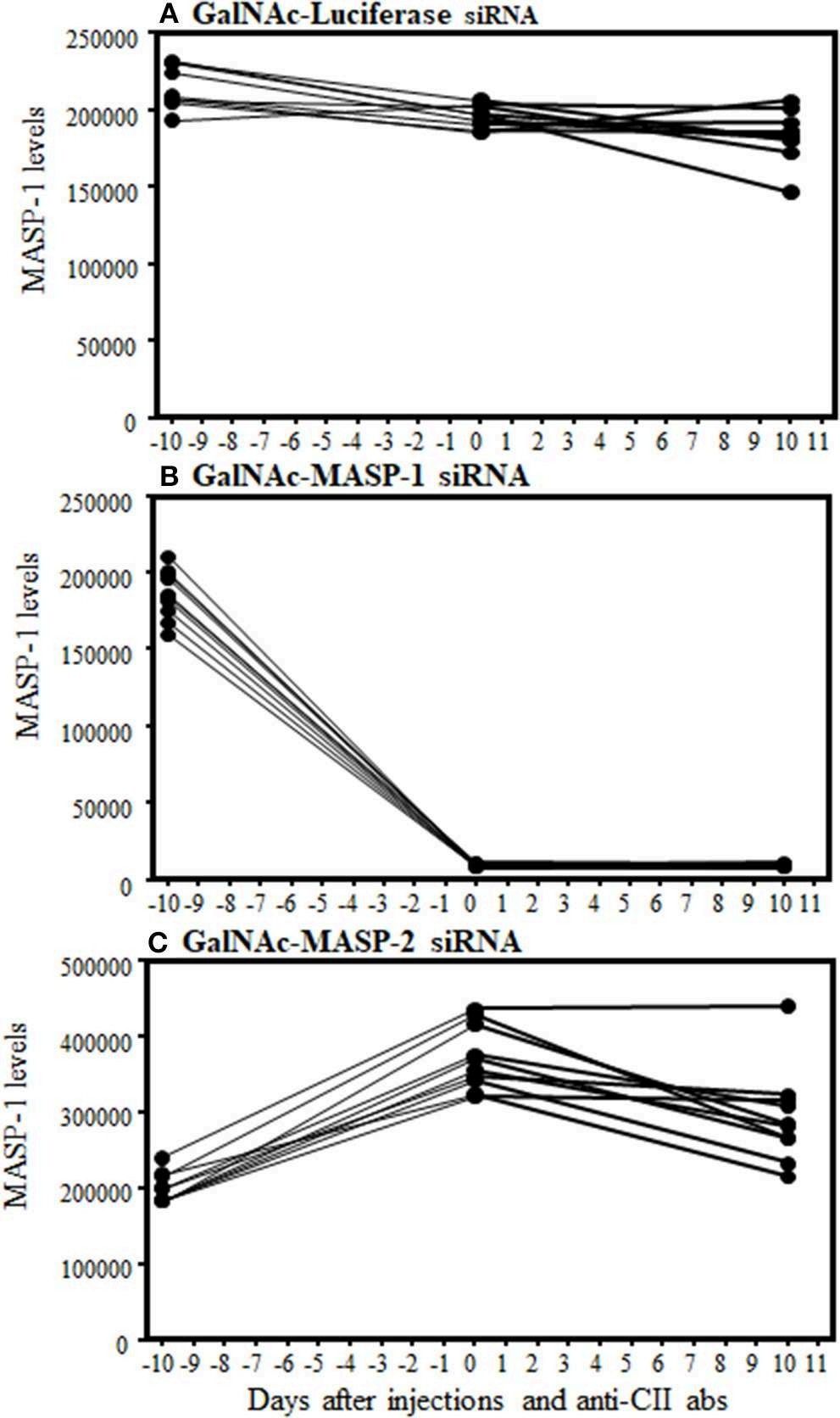 Fig.2 Time-course immunofluorometric assessment indicating reduced circulating MASP1 protein levels.2,3
Fig.2 Time-course immunofluorometric assessment indicating reduced circulating MASP1 protein levels.2,3
The role of the complement system in rheumatoid arthritis (RA) pathogenesis is significant, with MASPs not only driving lectin pathway activation but also regulating the alternative pathway. To explore the impact of MASP1 and MASP2 knockdown, researchers utilized N-acetylgalactosamine (GalNAc)-conjugated siRNAs in both in vitro settings and murine models of arthritis. In vitro, GalNAc-siRNAs effectively silenced MASP1 and MASP2 expression at picomolar concentrations. In an arthritis mouse model, administration of GalNAc-siRNAs prior to disease induction resulted in substantial liver-specific reductions of MASP mRNA and circulating protein levels, with MASP1-siRNA decreasing MASP1 by 95% and MASP2-siRNA achieving a 90% reduction in C4b deposition, pointing to their therapeutic potential in managing RA.
Creative Biolabs offers a range of specialized MASP1 services, including binding analysis and supplementary functional evaluations, tailored to support our valued clients in both clinical and research contexts.
References
-
Dobó, József, et al. "The Lectin Pathway of the Complement System—Activation, Regulation, Disease Connections and Interplay with Other (Proteolytic) Systems." International Journal of Molecular Sciences 25.3 (2024): 1566.
-
Holers, V. Michael, et al. "Key components of the complement lectin pathway are not only required for the development of inflammatory arthritis but also regulate the transcription of factor D." Frontiers in Immunology 11 (2020): 201.
-
Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig. 1 Structure of MASP1.1, 3
Fig. 1 Structure of MASP1.1, 3
 Fig.2 Time-course immunofluorometric assessment indicating reduced circulating MASP1 protein levels.2,3
Fig.2 Time-course immunofluorometric assessment indicating reduced circulating MASP1 protein levels.2,3
