Mannose Binding Lectin 2 (MBL2)
The complement lectin pathway, one of the three pathways that play important roles in both innate immunity and acquired immunity, is initiated by the binding of pattern recognition proteins to microbial surface carbohydrates. Mannose-binding lectin 2 (MBL2) is one of the central recognition proteins mediating the activation of the complement lectin pathway.
Human MBL2 (also known as mannose-binding protein C, MPB-C), a 26 kD O-glycosylated polypeptide with 248 amino acid residues, is a C-type lectin synthesized in liver cells. Each MBL2 molecule consists of four distinct domains: an N-terminal cysteine-rich domain, a collagen-like domain, an α-helical hydrophobic neck region, and a C-terminal carbohydrate-recognition domain. Three identical polypeptide chains form a triple helix subunit through the collagen-like region, which is stabilized by disulfide bonds within the N-terminal cysteine-rich region and oligomerization. MBL2 circulates in serum in an oligomeric tulip-like nanobouquet form since single MBL trimers are not fully functional.
MBL2 mediated complement lectin pathway activation starts with binding to carbohydrate structures on pathogenic organisms. MBL2 can not only selectively bind to mannose, but also recognize 3-hydroxy and 4-hydroxy carbohydrates on the equatorial plane of the sugar ring, such as L-fucose, N-acetylglucosamine, and N-acetylmannosamine. In human circulating, MBL2 usually is complexed with MBL-associated serum proteases (MASPs). Once MBL2 binds to sugar-rich microorganism surface, the MASPs, especially MASP-2, are activated, leading to the cleavage of complement C4 and C2. The C4b and C2a fragments covalently bind to the microbial surface, generating C3 convertase (C4b2a), which is pivotal to complement activation and amplification. Another important function of MBL2 is that it is capable of binding to late apoptotic and necrotic cells, senescent fibroblasts, inducing complement-dependent cytotoxicity. MBL2 deficiency has been associated with a higher risk of infections, immunodeficiencies, and pulmonary tuberculosis, while high MBL activity is related to autoimmune disease.
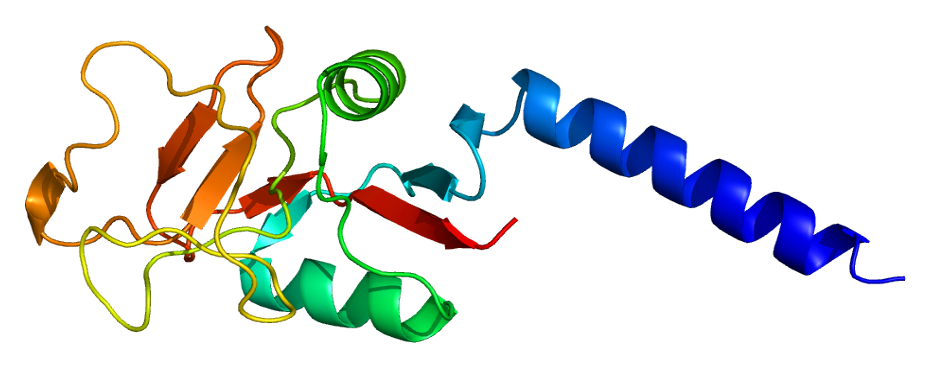 Fig.1 Structure of the mannose-binding lectin molecule. (Nuytinck, 2004)
Fig.1 Structure of the mannose-binding lectin molecule. (Nuytinck, 2004)
Reference
-
Nuytinck, L.; Shapiro, F. Mannose-binding lectin: laying the stepping stones from clinical research to personalized medicine. Personalized Medicine. 2004, 1(1): 35-52.
For Research Use Only.

 Fig.1 Structure of the mannose-binding lectin molecule. (Nuytinck, 2004)
Fig.1 Structure of the mannose-binding lectin molecule. (Nuytinck, 2004)