Product List Background CD46 Functional Service
Background
Membrane cofactor protein (MCP), also known as CD46 (cluster of differentiation 46), is a complement regulatory transmembrane glycoprotein that plays a crucial role in suppressing autoimmune responses and protecting host cells from complement-mediated damage. The MCP protein encoded by the CD46 gene has four isoforms, each of which contains four complement control protein (CCP) modules which contains the C3b and C4b binding sites and is responsible for cofactor activity. However, the four CCP modules differ in glycosylation and core proteins that extend from the cell membrane. MCP is expressed on all nucleated human cells such as T cells, B cells, natural killer cells, monocytes, neutrophils, platelets, endothelial cells, epithelial cells, fibroblasts, placenta, and sperm.
MCP acts as a cofactor for complement factor I, responsible for cleavage of C3b and C4b on host tissue, and protecting host cells from complement-mediated attack. It may also act as a costimulatory factor for T-cells, inducing the differentiation of CD4+ into T-regulatory 1 cells which suppress immune responses by secreting interleukin-10. In addition, MCP may be associated with the fusion of the spermatozoa with the oocyte during fertilization. Deficiencies of MCP are associated with atypical hemolytic uremic syndrome (aHUS) and many autoimmune diseases including multiple sclerosis (MS), rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE).
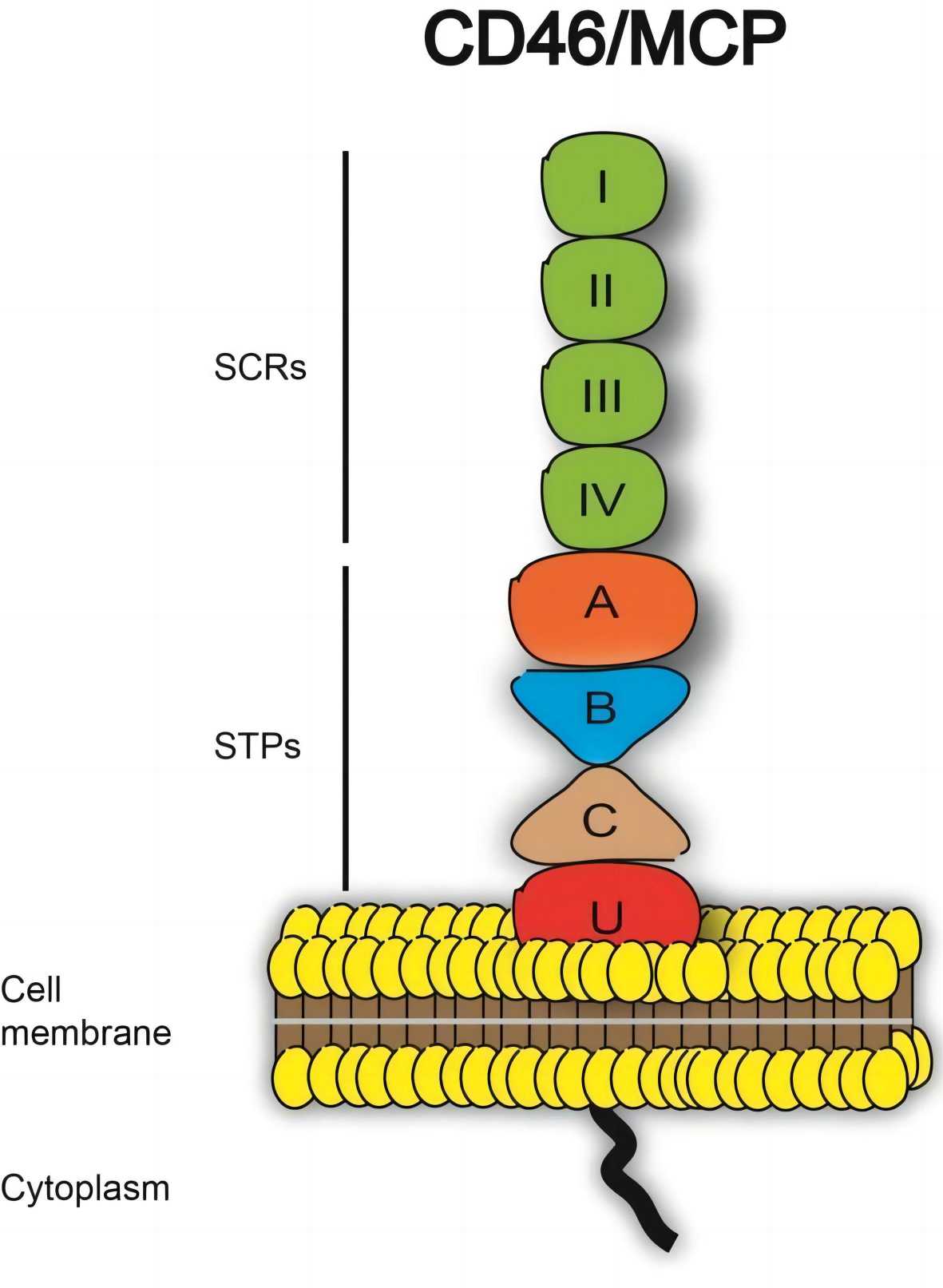 Fig. 1 Structure of CD46.1
Fig. 1 Structure of CD46.1
CD46 Functional Service
Creative Biolabs provides a comprehensive range of CD46-related products, encompassing anti-CD46 Antibodies, CD46 ELISA Assay kits, and recombinant CD46 Proteins with high specificity, sensitivity, and reproducibility, enabling accurate and reliable detection, quantification, and functional analysis of CD46.
CD46 functional tests are crucial for studying its role as a cofactor in complement regulation and its involvement in viral infections, such as those caused by measles and adenovirus. Functional tests can be performed using various methods, including ELISA, flow cytometry, and cellular assays. These tests conducted using our CD46 products can evaluate the binding of anti-CD46 antibodies, CD46's cofactor activity, and its interaction with pathogens or complement components.
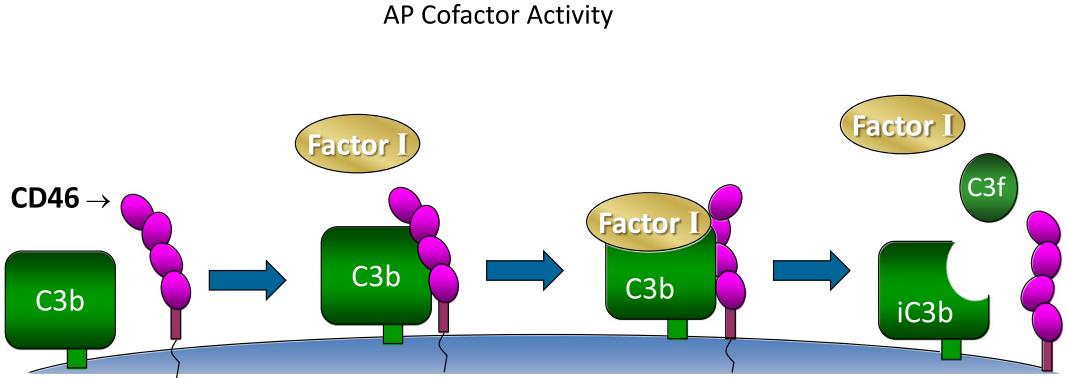 Fig.2 The cofactor activity of CD46.2
Fig.2 The cofactor activity of CD46.2
For instance, a study has evaluated the clinical utility of serum soluble CD46 as a marker of disease activity in patients with SLE by measuring serum levels of sCD46 by ELISA using two different anti-human CD46 monoclonal antibodies. Another study utilizing CD46 blocking peptides has demonstrated the inhibition of adenovirus infection in human cells, highlighting CD46's importance as a viral receptor. Thus, functional assays have helped identify therapeutic strategies targeting CD46 for complement-related diseases and viral infections.
Creative Biolabs provides CD46-functional services, including custom functional assays, receptor-binding studies, and complement activation tests. Our services empower clients with accurate data and expert guidance, accelerating research and development in immunology and infectious diseases.
References
-
Delpeut, Sebastien, Ryan S. Noyce, and Christopher D. Richardson. "The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses." Viruses 6.6 (2014): 2268-2286. Distributed under Open Access license CC BY 3.0. The image was modified by extracting and using only part of the original image.
-
Liszewski, M. Kathryn, and John P. Atkinson. "Complement regulator CD46: genetic variants and disease associations." Human genomics 9 (2015): 1-13. Distributed under Open Access license CC BY 4.0, without modification.


 Datasheet
Datasheet Fig. 1 Structure of CD46.1
Fig. 1 Structure of CD46.1
 Fig.2 The cofactor activity of CD46.2
Fig.2 The cofactor activity of CD46.2
