Quantifying the binding parameters of aptamer-ligand pairs is an important part of characterizing the function of aptamers. Isothermal Titration Calorimetry (ITC) is a general technique for conducting combined studies because it is easy to perform a series of experiments under many different solution conditions.
Thermodynamic Profiling for Aptamer Characterization
Aptamers are nucleic acids with high affinity and specificity selected from systematic evolution of ligands by exponential enrichment (SELEX). Due to the large diversity of aptamer structures, it is necessary to understand its mechanism of action, which will contribute to the development of its therapeutic applications. Revealing the recognition mechanism of aptamers to target molecules is generally carried out through biophysical tools and structural studies. ITC has been used to develop small therapeutic agents that bind to target proteins, and is increasingly used to study aptamer-target protein interactions.
Introduction of ITC
ITC benefits from a non-destructive and label-free technology that provides the stoichiometry of interaction and a set of thermodynamic binding parameters: equilibrium binding constant (Ka or Kd) and changes in enthalpy (∆H) and entropy (∆S). Due to the increased sensitivity of ITC instruments in the past 10 years, fewer sample volumes are now required, making it easier to use in biology, including aptamers that bind to target proteins. When this binding occurs, heat is usually released or absorbed, which is usually measured by gradually titrating the ligand into the target molecule sample in the ITC cell with a calorimeter. The integration result of the heat pulse can be plotted as a function of the molar ratio to form a titration curve. The binding stoichiometric ratio (N), Kd and ∆H can be obtained from this titration curve.
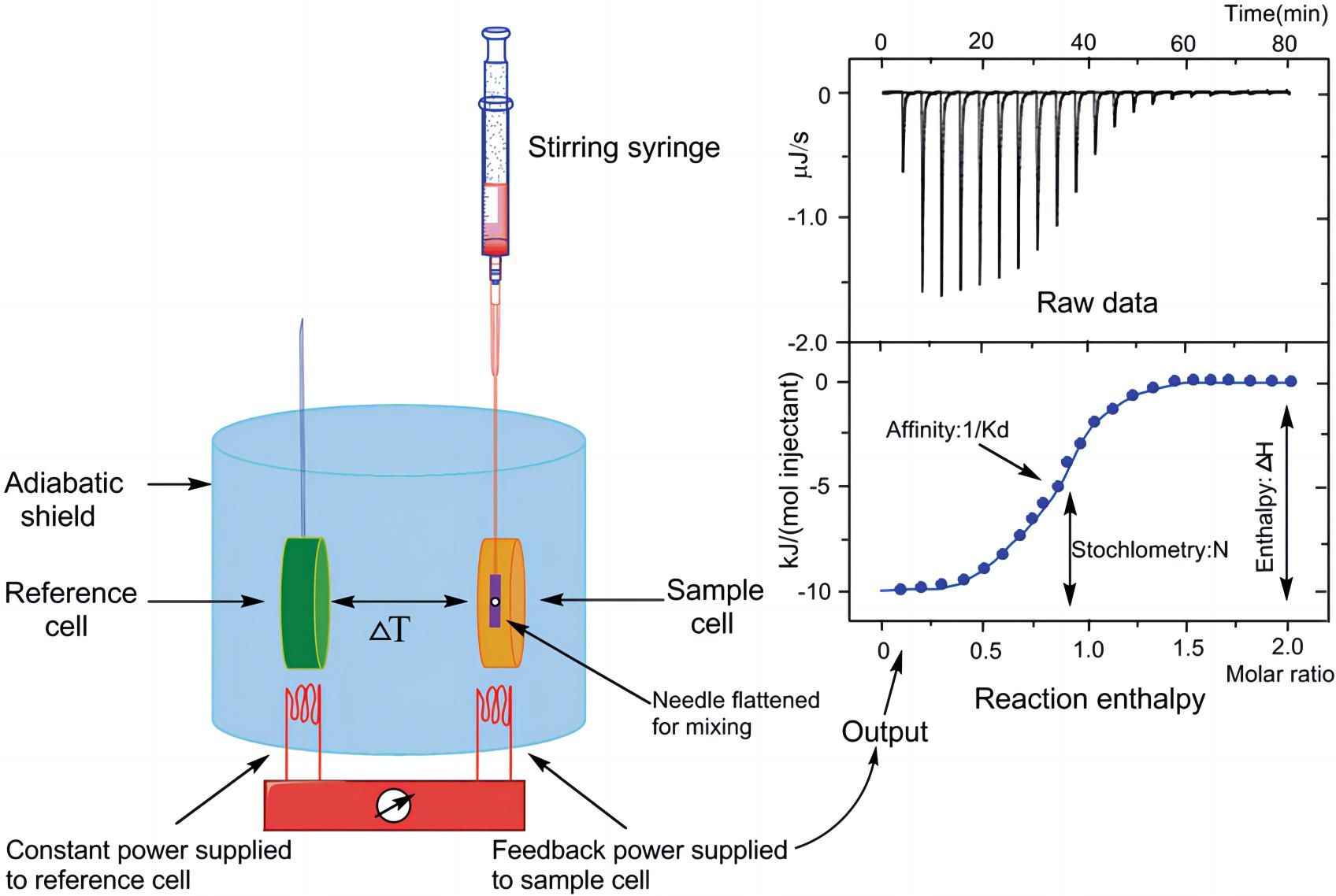 Fig. 1 A schematic diagram of ITC experiments.1, 2
Fig. 1 A schematic diagram of ITC experiments.1, 2
Services in Creative Biolabs
Recent advances have been made in developing and improving instruments to analyze biophysical aspects of biomolecules. A combination of structural and thermodynamic studies should accelerate the structure-based development of aptamers. ITC is a fast and reliable method to study the physical basis of molecular interaction. In the case of a single experiment, solution and unlabeled, the full thermodynamic profile of molecular interaction can be obtained, so aptamers can be evaluated and optimized more effectively. This method can be used to:
-
In-depth characterization of aptamer-target molecular interaction
-
Ranking of inhibitors or candidate drugs in drug discovery
-
Effectiveness assessment of aptamer modification
Creative Biolabs uses ITC technology to provide comprehensive and accurate analysis services for aptamer molecular interactions. You can combine our ITC services to solve all problems in drug discovery and aptamer characterization. If you are interested in our service, please contact us for your exclusive solution.
References
-
Song, Chengcheng, Shaocun Zhang, and He Huang. "Choosing a suitable method for the identification of replication origins in microbial genomes." Frontiers in microbiology 6 (2015): 1049.
-
under Open Access license CC BY 4.0, without modification
Related Product
Questions & Answer
A: Key parameters include ΔH (enthalpy change), ΔS (entropy change), and ΔG (Gibbs free energy change), which collectively describe the aptamer-target interaction.
A: By understanding the thermodynamics of aptamer-target interactions, researchers can modify aptamer sequences to enhance binding affinity and specificity, resulting in improved aptamer performance.
A: Yes, we provide thermodynamic profiling of aptamers for various biotechnology and research companies. These services can include the use of advanced equipment and expertise to analyze aptamer-target interactions in detail.
For Research Use Only.
Related Sections:

 Fig. 1 A schematic diagram of ITC experiments.1, 2
Fig. 1 A schematic diagram of ITC experiments.1, 2