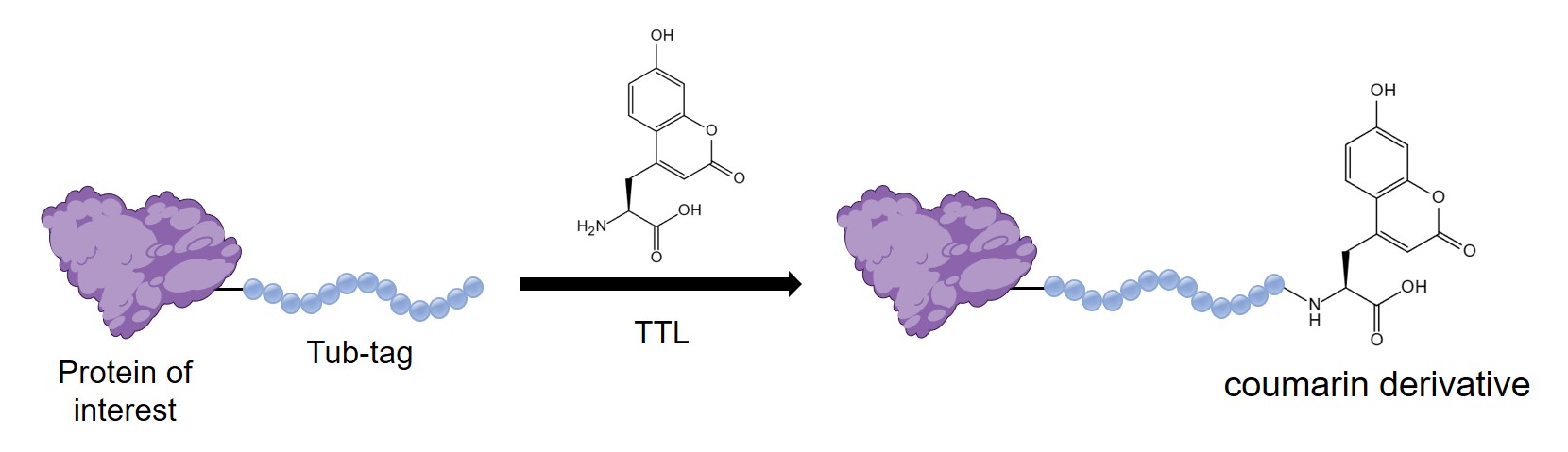
Chemoenzymatic Labeling with Tubulin Tyrosine Ligase
Tubulin Tyrosine Ligase (TTL) plays a pivotal role in the field of selective protein functionalization by facilitating the addition of tyrosine derivatives to the C-terminal carboxylic acids of proteins. This enzyme specifically recognizes the Tub-tag peptide sequence (VDSVEGEGEEEGEE), enabling the targeted conjugation of diverse tyrosine derivatives to proteins that incorporate the Tub-tag. The following method outlines an efficient one-step labeling approach for Tub-tag-fused target proteins utilizing coumarin derivatives under TTL catalysis.
Disclaimer
The procedures outlined in this document are for reference only. Creative Biolabs makes no assurances or warranties regarding the outcomes that may result from the customer's implementation of this guideline.
Materials
 Expression and Purification of TTL and Tub-Tagged Protein
Expression and Purification of TTL and Tub-Tagged Protein
- BL21(DE3) chemically competent E. coli cells.
- Plasmid encoding TTL.
- Plasmid encoding Annexin V-Tub-tag.
- LB medium (liquid broth).
- LB agar plates (supplemented with 100 μg/mL ampicillin).
- Stock solutions: ampicillin (100 mg/mL in water), isopropyl-β-D-thiogalactopyranoside (IPTG, 1 M in water), phenylmethanesulfonyl fluoride (PMSF, 0.2 M in isopropanol).
- Immobilized metal chelate affinity chromatography (IMAC) wash buffer of TTL (0.5 M NaCl, 50 mM Tris-HCI (pH 7.4), 10 mM dithiothreitol (DTT)).
- IMAC resuspension buffer of TTL (PMSF stock solution (100 μL, 0.2 M in isopropanol), 25 μg/mL DNAse, 100 μg/mL lysozyme).
- IMAC elution buffer of TTL (0.5 M NaCl, 500 mM imidazole, 50 mM Tris-HCl (pH 7.4), 10 mM DTT).
- Protein IMAC buffer (0.5 M NaCl, 50 mM Tris-HCI (pH 7.4)).
- Protein IMAC resuspension buffer (PMSF stock solution (100 μL, 0.2 M in isopropanol), 25 μg/mL DNAse, 100 μg/mL lysozyme).
- Protein IMAC elution buffer (0.5 M NaCl, 500 mM imidazole, 50 mM Tris-HCl (pH 7.4)).
- Buffer 1 (pH 7.0, 100 mM KCI, 20 mM MES-KOH, 10 mM MgCl2, for protein storage and ligation).
- Centrifugation tubes.
- Ni-NTA column.
- Desalting column.
 Conjugation of Coumarin-Derivative 1 to Tub-Tagged Protein by TTL
Conjugation of Coumarin-Derivative 1 to Tub-Tagged Protein by TTL
- Stock solutions: adenosine triphosphate (ATP, 100 mM in water), coumarin-derivative 1 (11 mM in buffer 1), DTT (2 M in water).
- Potassium hydroxide (KOH, 1N).
- KOH (0.1 N).
- Dialysis tube (1 kDa MWCO).
- Microcentrifuge tube (1.5 mL).
Methods
 Expression and Purification of TTL
Expression and Purification of TTL
- Thaw 50 μL of BL21 (DE3) on ice. Add the plasmid (1 μL, encoding TTL) to the cytosol and incubate the mixture on ice for 30 minutes. Then, perform a heat shock by placing the cells at 42°C for 45 seconds. Finally, add prewarmed LB medium (500 μL) and incubate the mixture at 37°C for 1 hour with continuous shaking (180 rpm).
- Carefully streak the transformed cell suspension (100 μL) onto an LB agar plate (supplemented with 100 μg/mL ampicillin). Then, incubate the agar plates on a shaker at 37°C overnight.
- Pick a single colony and inoculate 5 mL of LB medium (supplemented with 100 μg/mL ampicillin). Culture in an incubator shaker (180 rpm) overnight at 37°C.
- Add 2 mL of the culture into 250 mL of fresh sterile LB medium (supplemented with 100 μg/mL ampicillin), and then culture at 37°C in an incubator shaker (180 rpm) until the optical density at 600 nm (OD600) reaches 0.7.
- Add 125 μL of IPTG (1 M in water) into the culture to induce TTL expression. Then, incubate in an incubator shaker (180 rpm) at 18°C for 18 hours.
- Centrifuge (18,000 × g) the culture at 4°C for 30 minutes and resuspend the pellet in IMAC resuspension buffer of TTL (10 mL).
- Disrupt the cells using an ultrasonic cell disruptor with short ultrasonic pulses for three consecutive cycles of 90 seconds each. Perform the entire procedure on ice and make sure that the solution is cool before the next ultrasonic pulse.
- Centrifuge (49,000 × g) the cells at 4°C for 30 minutes to separate cellular debris. Then, apply the lysate directly to the Ni-NTA column via an FPLC system.
- To ensure effective purification, wash the column thoroughly with IMAC wash buffer of TTL (10 column volumes). Following this step, elute the immobilized Ho-TTL via gradually increasing the percentage of IMAC elution buffer of TTL to 100% within 2 column volumes.
- Assess the purity of eluted protein fractions via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. Subsequently, combine pure protein fractions and use protein desalting columns and buffer 1 to desalt them.
- Freeze TTL aliquots of 20 μL each at approximately 2 mg/mL in liquid nitrogen and store the aliquots at -80°C.
 Expression and Purification of Tub-Tagged Protein
Expression and Purification of Tub-Tagged Protein
- Transfect the plasmid encoding Annexin V-Tub-tag using the method in “Expression and purification of TTL” (steps 1 ~ 4).
- Add 125 μL of IPTG (1 M in water) into the culture to induce Tub-tagged protein expression. Then, incubate in an incubator shaker (180 rpm) at 37°C for 5 hours.
- Centrifuge (18,000 × g) the culture at 4°C for 30 minutes and resuspend the pellet in protein IMAC resuspension buffer (10 mL).
- Disrupt the cells using an ultrasonic cell disruptor with short ultrasonic pulses for three consecutive cycles of 90 seconds each. Perform the entire procedure on ice and make sure that the solution is cool before the next ultrasonic pulse.
- Centrifuge (49,000 × g) the cells at 4°C for 30 minutes to separate cellular debris. Then, apply the lysate directly to the Ni-NTA column via an FPLC system.
- To ensure effective purification, wash the column thoroughly with protein IMAC wash buffer (10 column volumes). Following this step, elute the immobilized Ho-TTL by gradually increasing the percentage of protein IMAC elution buffer to 100% within 2 column volumes.
- Assess the purity of eluted protein fractions via SDS-PAGE analysis. Subsequently, combine pure protein fractions and use protein desalting columns and buffer 1 to desalt them.
- Freeze protein-Tub-tag aliquots of 20 μL each at approximately 4 mg/mL in liquid nitrogen and store the aliquots at -80°C.
 Conjugation of Coumarin-Derivative 1 to Tub-Tagged Protein by TTL
Conjugation of Coumarin-Derivative 1 to Tub-Tagged Protein by TTL
- Add 100 μL of buffer 1 into a microcentrifuge tube (1.5 mL).
- Add ATP stock solution (5 μL), coumarin-derivate 1 stock solution (13.64 μL), and DTT stock solution (0.38 μL). Their final concentrations are 2.5 mM, 1 mM, and 5 mM, respectively.
- Adjust the pH of the mixture to 7.0 using 1 N and 0.1 N KOH.
- Add buffer 1 to bring the total volume to 134.72 μL.
- Add protein-Tub-tag solution (11.68 μL) and TTL solution (3.6 μL) to a final concentration of 10 μM and 1 μM, respectively.
- Incubate the mixture in a dry block incubator at 37°C for 1 ~ 3 hours.
- Remove excess coumarin-derivate 1 and reducing agent by dialyzing the mixture at 4°C for 3 hours with a dialysis tube (1 kDa MWCO) against buffer 1. Store the fluorescent proteins at 4°C.
Notes
- E. coli strains equipped with the T7 polymerase promoter system are recommended for high-level gene expression and regulation.
- To form single colonies, it is necessary to ensure that the cell suspension is uniformly streaked.
- The alteration of the cell suspension to a gray hue during ultrasonic treatment indicates the successful destruction of the cells.
- If the purity is insufficient after His-tag purification, subsequent size exclusion chromatography using Buffer 1 is recommended. Then assess the purity of the eluted protein fractions by SDS-PAGE analysis and combine the purified fractions.
- To achieve the desired pH of the mixture, the initial treatment involves the addition of 1 N KOH, bringing the pH to approximately 7.0. Following this step, 0.1 N KOH is used for the final adjustment of the pH.
- Do not shake while incubating in the conjugation reaction to prevent protein aggregation.
